What we do
The COVID-NMA process is based on three pillars: a living mapping of registered trials, a living systematic review of trial results and a living monitoring of trials transparency.
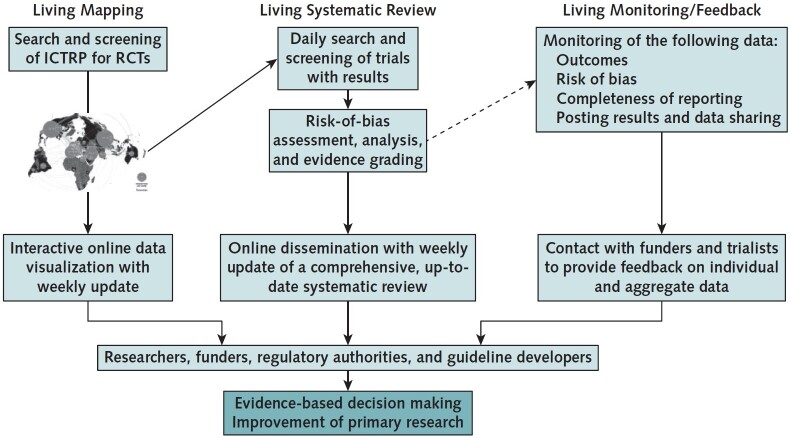
Source: https://www.acpjournals.org/doi/10.7326/M20-5261
Living mapping of registered trials
- We are searching the WHO International Clinical Trials Registry Platform (ICTRP) once a week to identify and extract data from RCTs evaluating the effectiveness of interventions for preventing and treating COVID-19 as well as of all trials assessing vaccines.
- This feeds into two living data visualizations of treatments and vaccines developed jointly with the LIRIS Laboratory (CNRS/École Centrale de Lyon) in the context of a large collaboration with the CNRS. These are updated once a week. Studies can be filtered, for example, by their status, country, design, registration date, type of treatment or vaccine being studied.
Living synthesis of trial results
- We are searching the L.OVE platform and the the Cochrane COVID-19 Study register platform every day to identify new RCTs with results.
- We collect data from all RCTs identified, including their main characteristics and a complete description of the assessment of each risk of bias domain with support for judgement, and incorporate this into our evidence synthesis.
- We present results through forest plots, the Evidence profile and Summary of Findings (SoF) tables, that are updated once a week.
- We contact authors of published studies to request for additional information, when needed, in order to accurately include their study in our evidence synthesis.
- We contact investigators of ongoing trials to obtain the trial protocol and results as soon as available.
- Where appropriate we will undertake network meta-analysis to synthesize the available study results and compare simultaneously all possible interventions that could be used in the same clinical scenario.
Living monitoring of trial transparency
We are assessing the trials’ methodological quality and transparency in order to provide trialists with an individual feedback and community aggregated results. We hope this will help improve the reporting of future COVID-19 trials.
Publications
15 articles have been published in scientific journals. See more