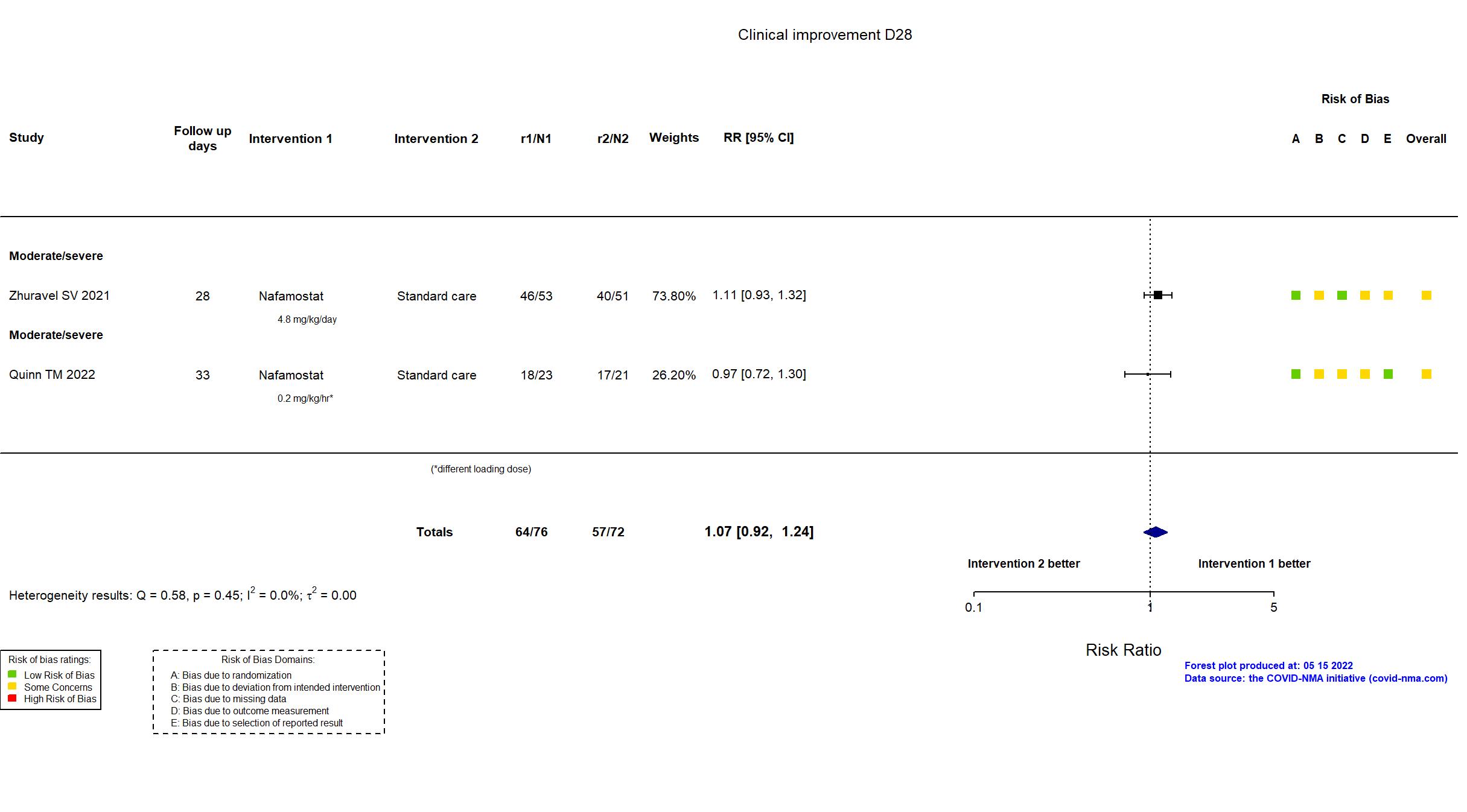
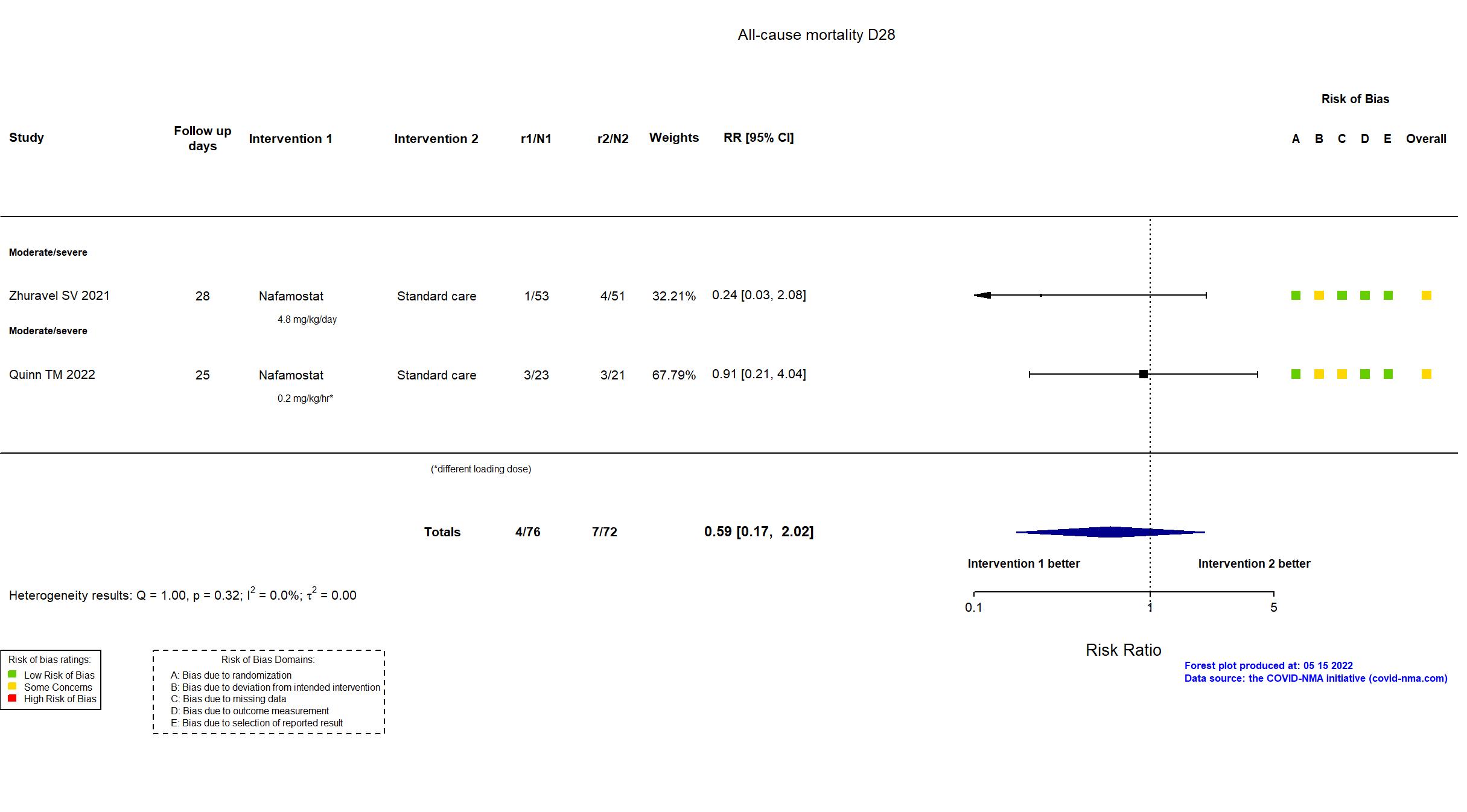
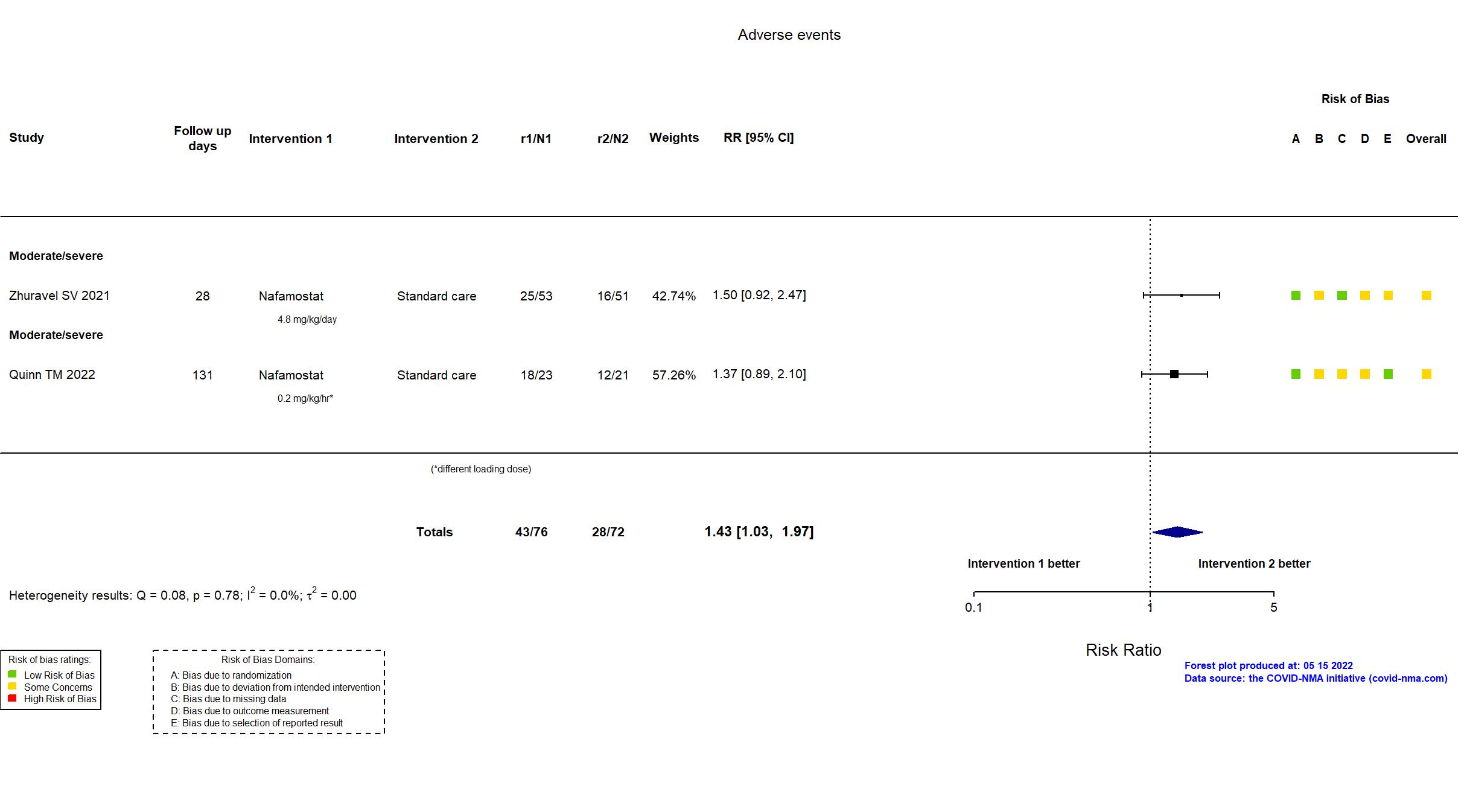
Nafamostat vs Standard care (RCT)
Hospitalized patients
FOREST PLOTS -2022-05-16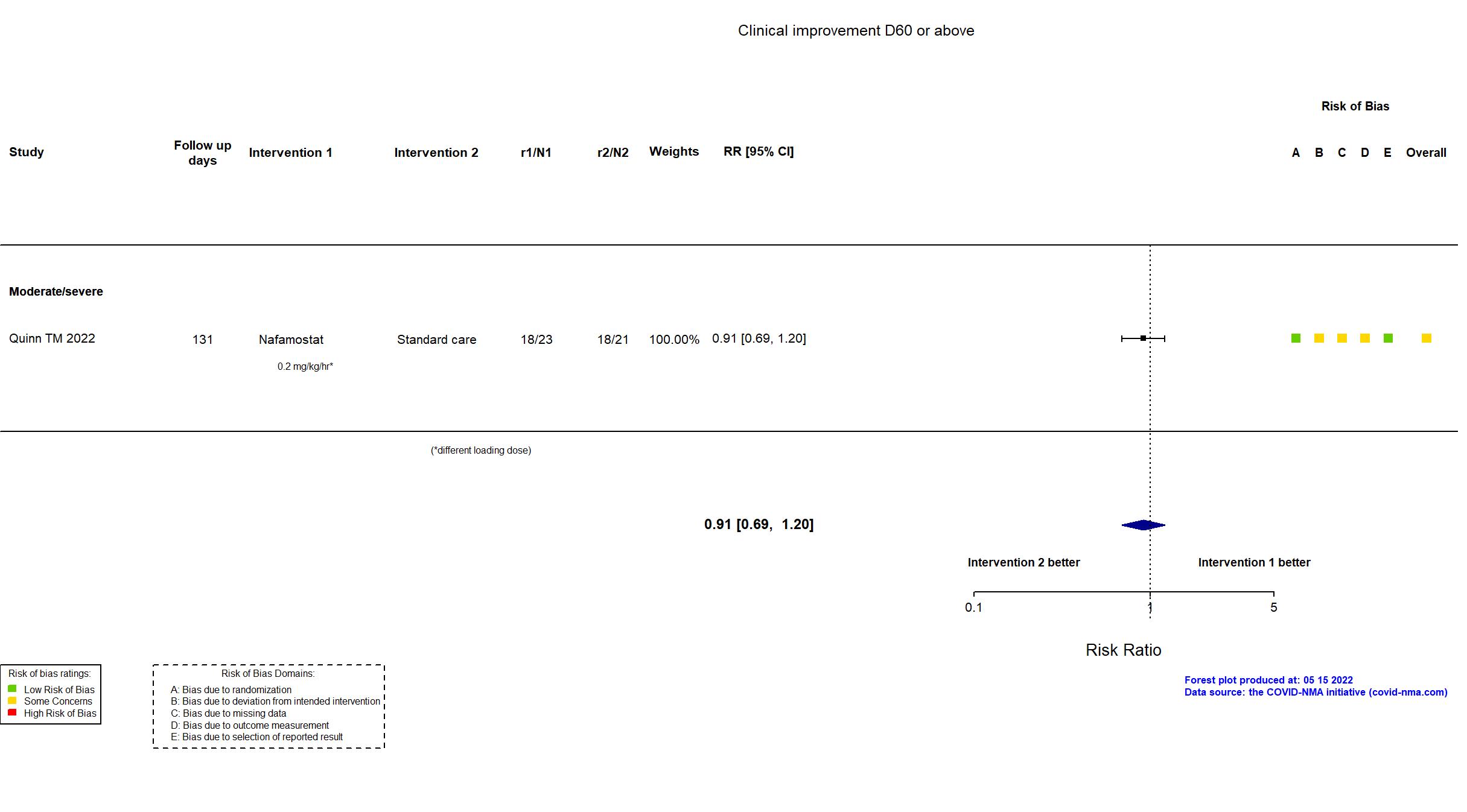
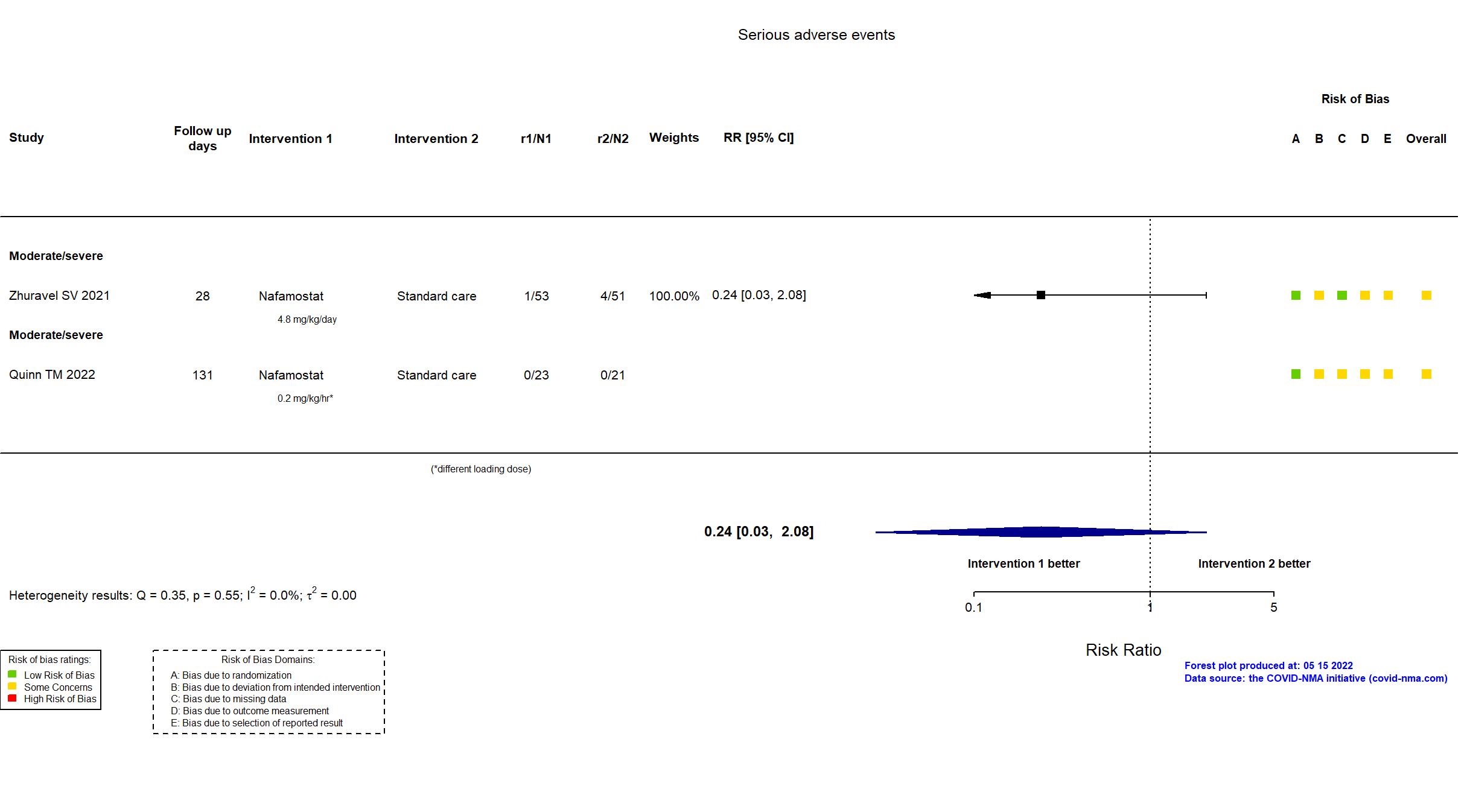
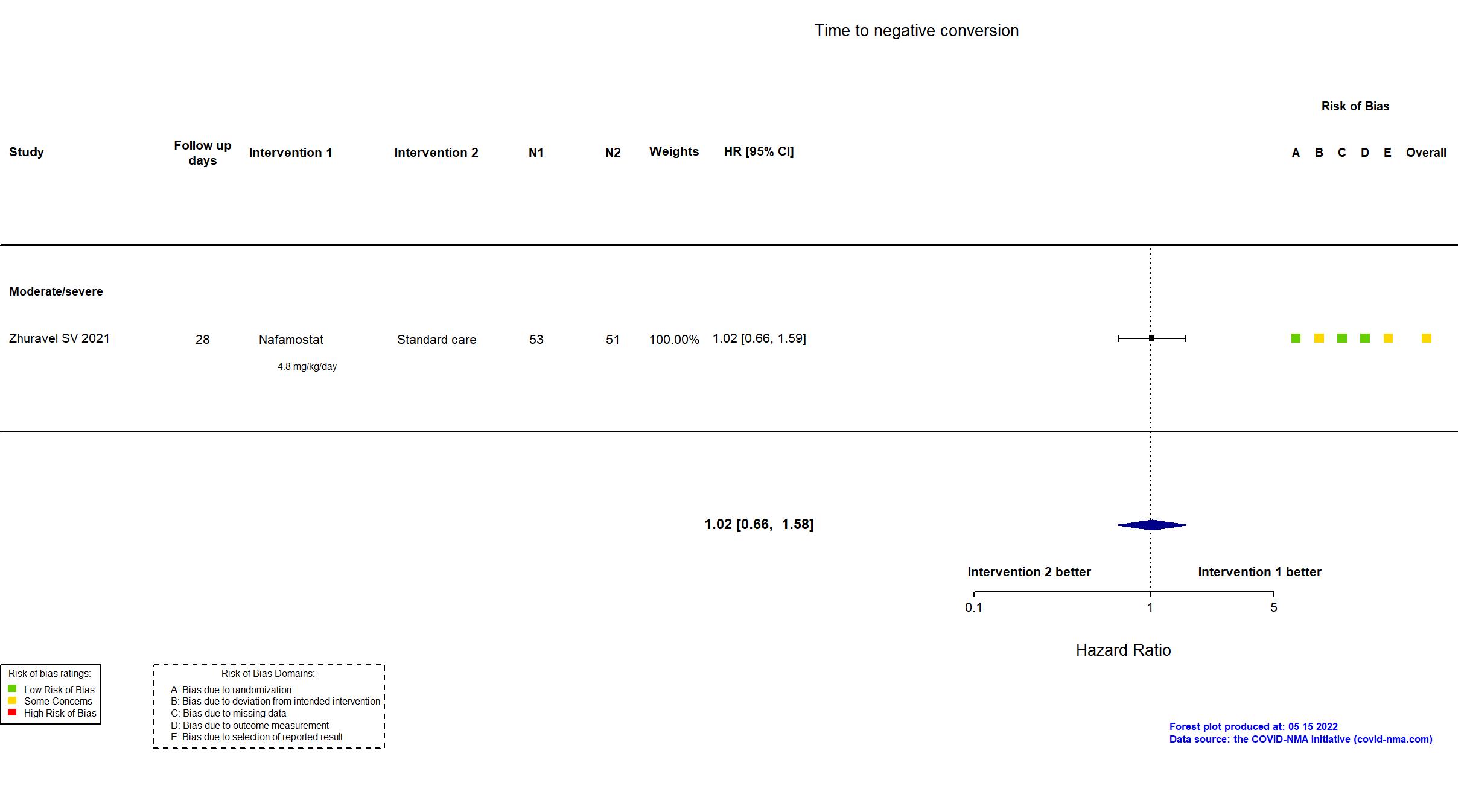






Trial NCT04473053; ISRCTN14212905; EudraCT 2020-002230-3
Publication DEFINE - Quinn TM, EBioMedicine (2022) (published paper)
Dates: 2020-09-01 to 2021-02-28
Funding: Mixed (LifeArc and Oxford
University COVID-19 Research Response Fund. Nichi-Iko (Japan) supplied Futhan (intravenous Nafamostat) for the trial.)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / UK Follow-up duration (days): 131 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Nafamostat 0.2 mg/kg/hr continuous infusion for 7 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Nafamostat=23 Standard care=21 | |
| Characteristics of participants N= 44 Mean age : NR 25 males Severity : Mild: n=0 / Moderate: n=* / Severe: n=* Critical: n=0 | |
| Primary outcome | |
| In the register 1) The safety of the candidate therapies in COVID-19 patients by measuring physiological changes in the circulatory and respiratory system. [ Time Frame: Up to 16 days post treatment ]; 2) The safety of the candidate therapies in COVID-19 patients by recording the number of treatment related adverse events. [ Time Frame: Up to 90 days post treatment ] | |
| In the report safety and tolerability of intravenous nafamostat as an add on therapy for patients hospitalised with COVID-19 pneumonitis. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the pre-print and published articles, the protocol and study registries were used in data extraction and risk of bias assessment. The primary outcomes in the article broadly reflect those in the registry. The two arms in the platform trial reported in the article along with a third unreported arm (n = 66 in total) appear not to have achieved the target sample size in the registry (n = 200).
This study was updated on May 11th, 2022 with data extracted from the peer-reviewed report. |
Trial NCT04623021
Publication Zhuravel SV, EClinicalMedicine (2021) (published paper)
Dates: 2020-09-25 to 2020-11-14
Funding: Mixed (Korea Research Institute of Bioscience and Biotechnology; Chong Kun Dang (CKD) Pharm (Seoul, Korea).)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Russia Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Nafamostat 4.8 mg/kg/day intravenously for 10 days or until hospital discharge. Dose reduction was allowed by 0.1 mg/kg/hr (2.4 mg/kg/day) at the investigator's discretion as indicated by patient tolerability. |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Nafamostat=53 Standard care=51 | |
| Characteristics of participants N= 104 Mean age : NR 51 males Severity : Mild: n=0 / Moderate: n=94 / Severe: n=9 Critical: n=0 | |
| Primary outcome | |
| In the register Time to clinical improvement [ Time Frame: up to 28 days ] Time to clinical improvement (TTCI) was defined as time (days) from randomization to a decline of 2 categories on the seven-category ordinal scale of clinical status or live discharge from the hospital, whichever came first | |
| In the report Time to clinical improvement defined as the time from randomisation to either discharge from hospital or improvement of two points on the 7-category ordinal scale, whichever came first. | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the retrospective trial registry and supplementary appendices were used in data extraction and assessment of risk of bias. There is no information whether the sample size, outcomes or analysis plan were determined a-priori. The trial (n = 104) achieved the target sample size in the first version of the registry (n = 100). |