Intravenous Immunoglobulin vs Standard care/Placebo (RCT)
Hospitalized patients
Studies included but not extracted/included in the analysis: NCT04480424 results posted on clinicaltrials.gov
FOREST PLOTS -2022-04-29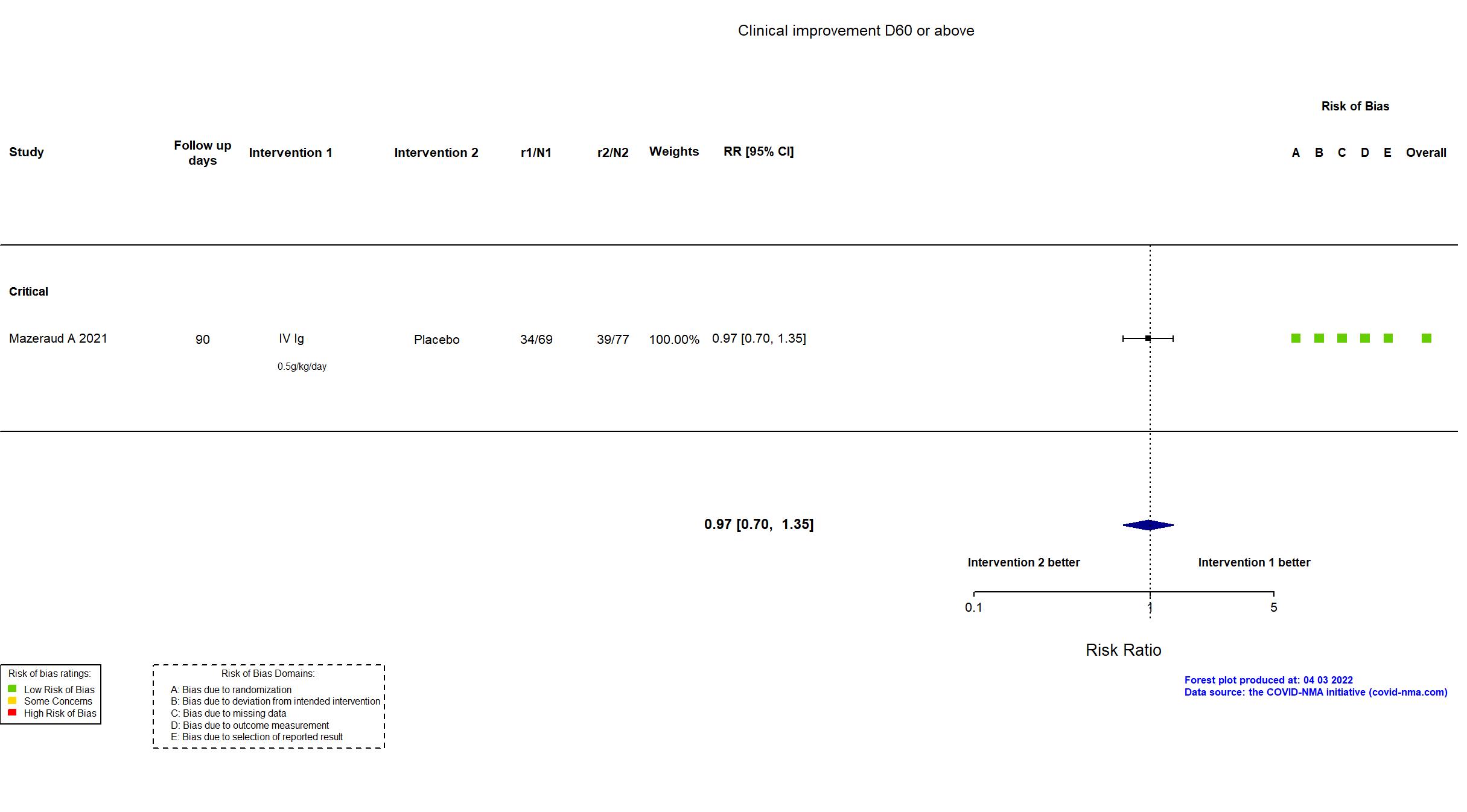
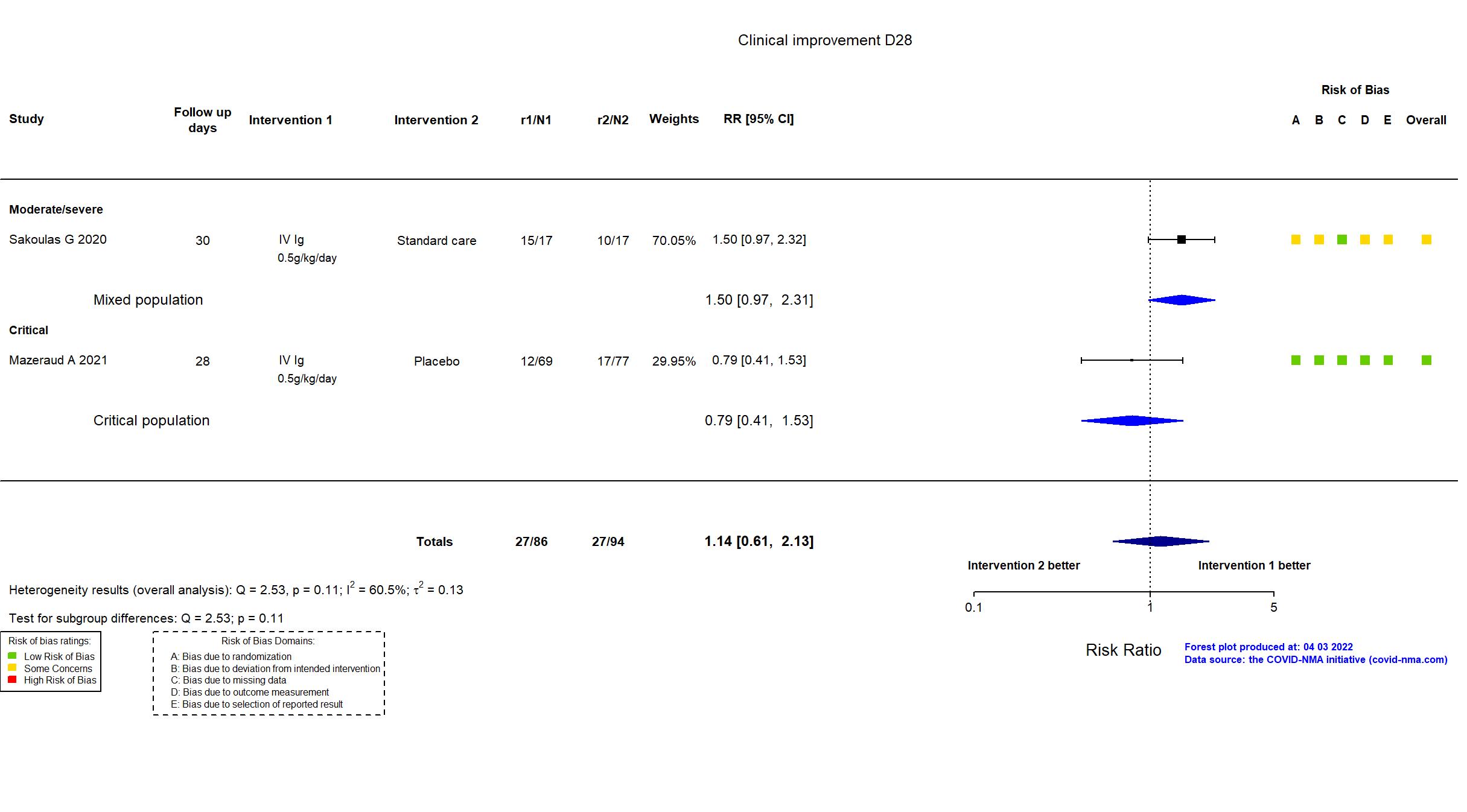
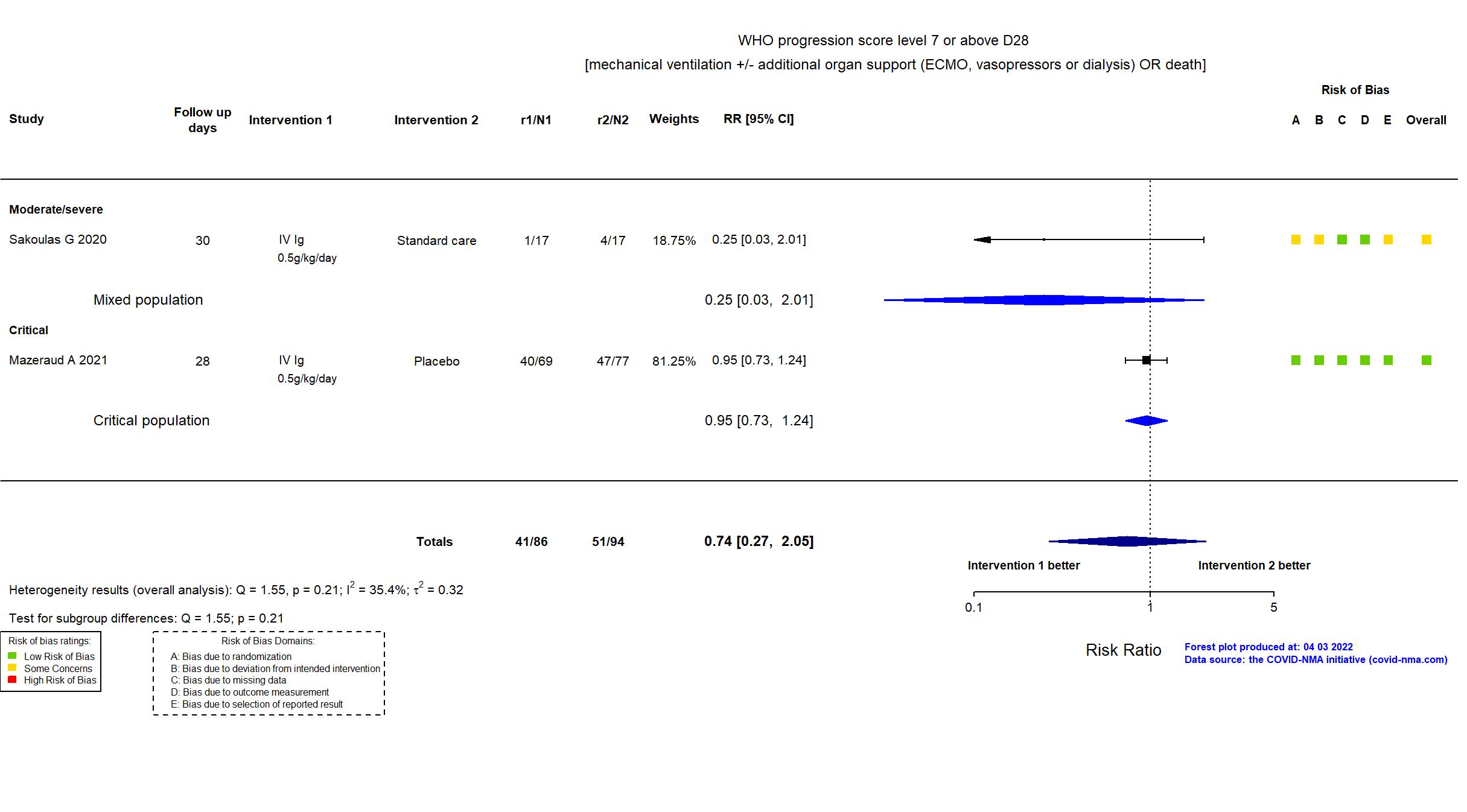
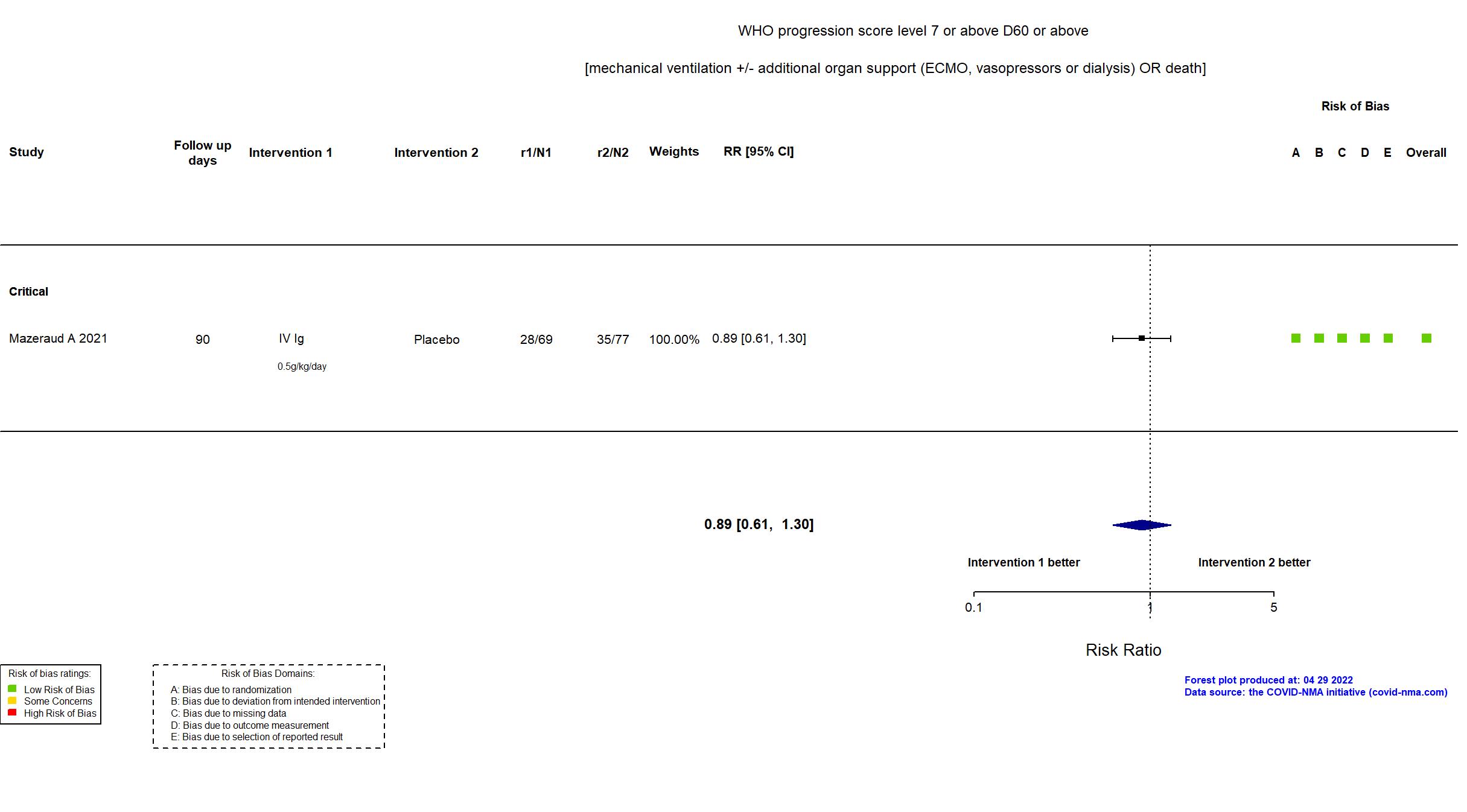
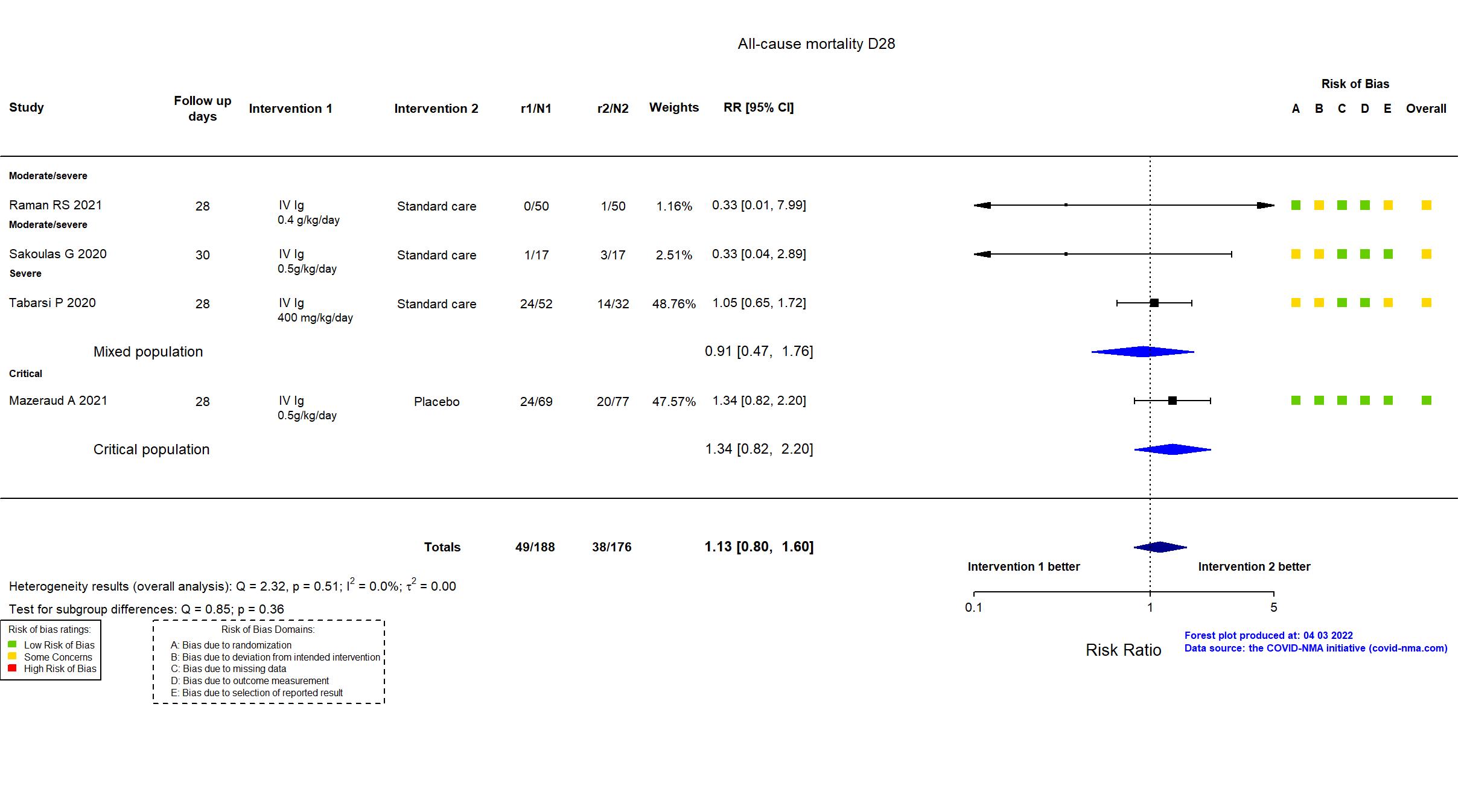
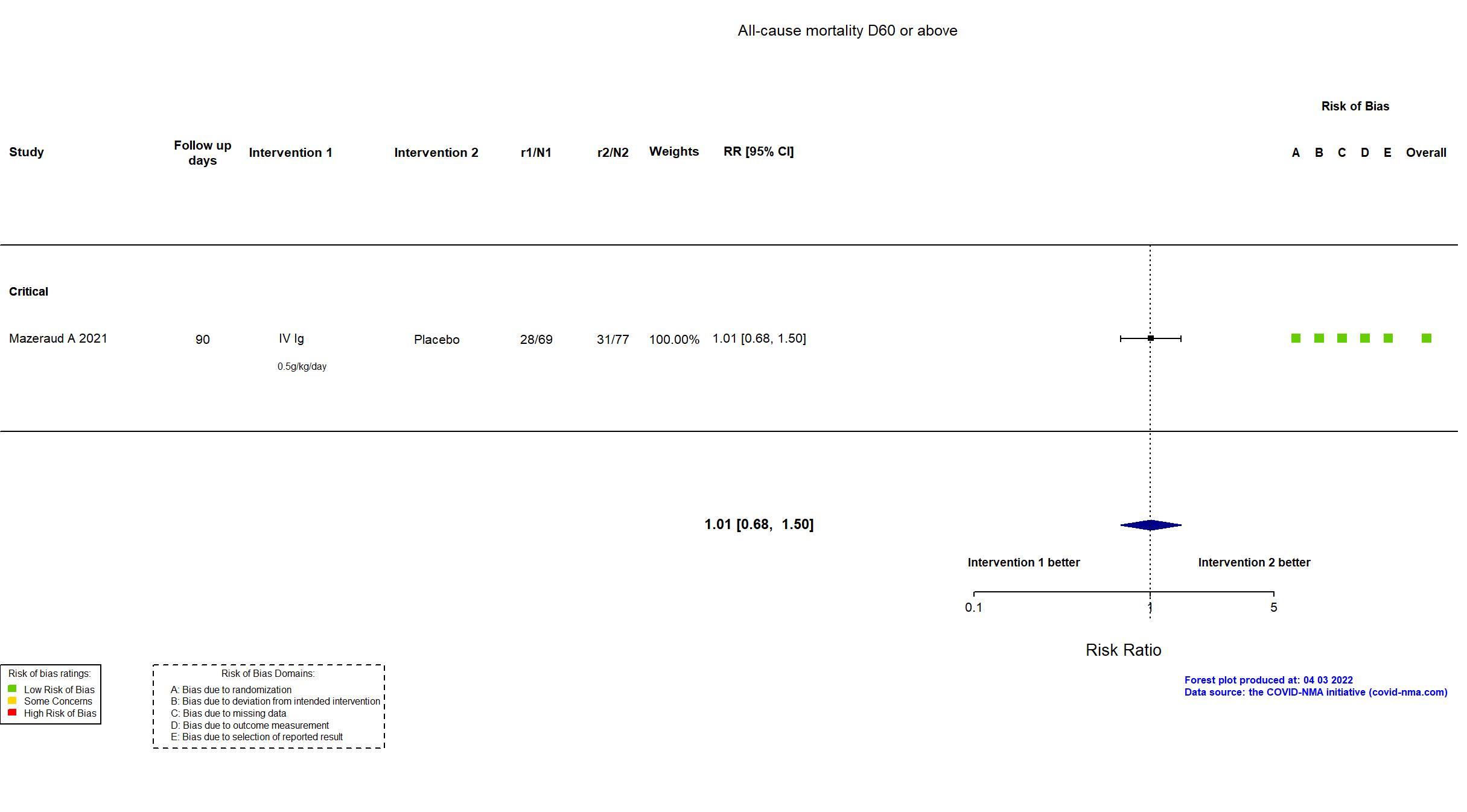

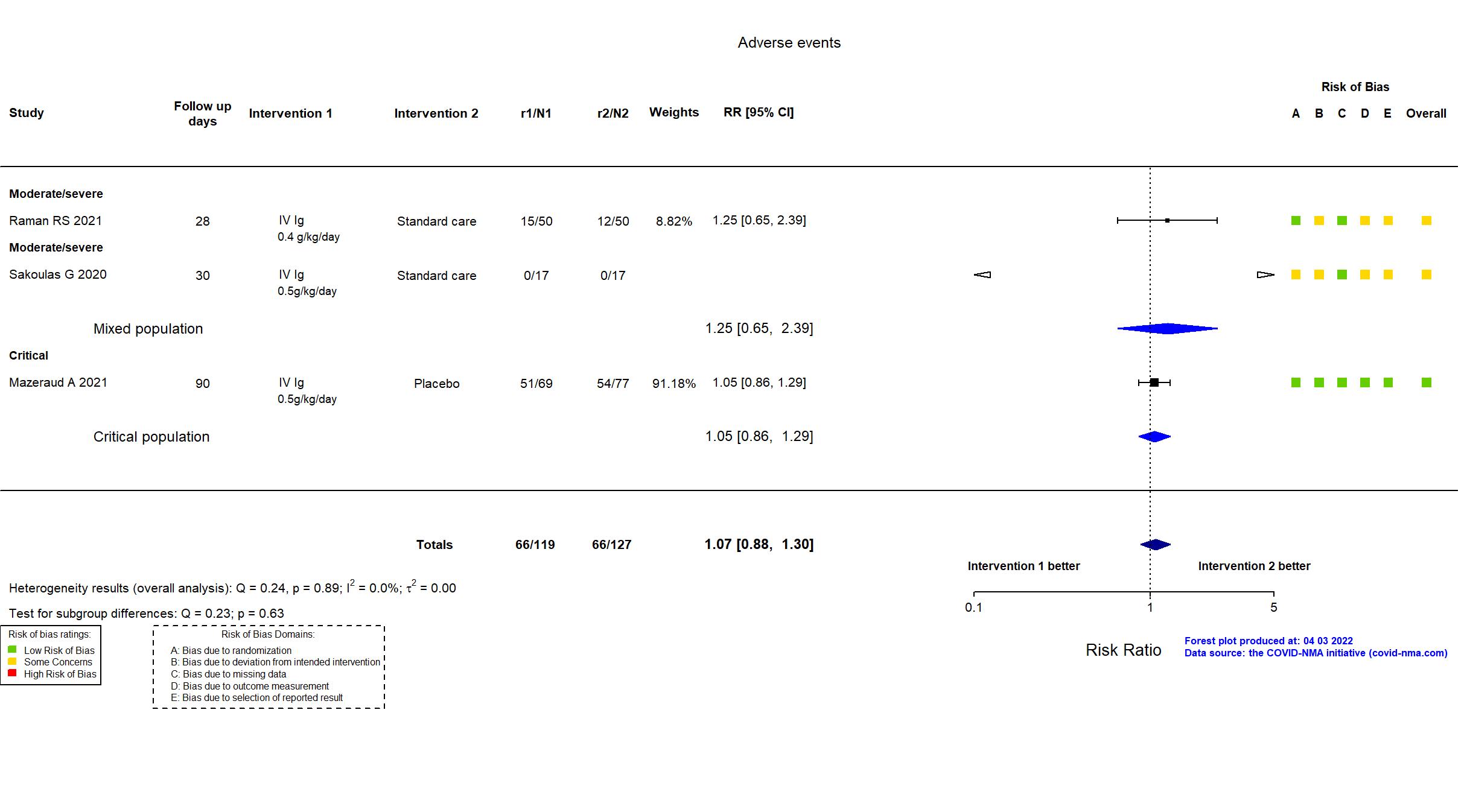
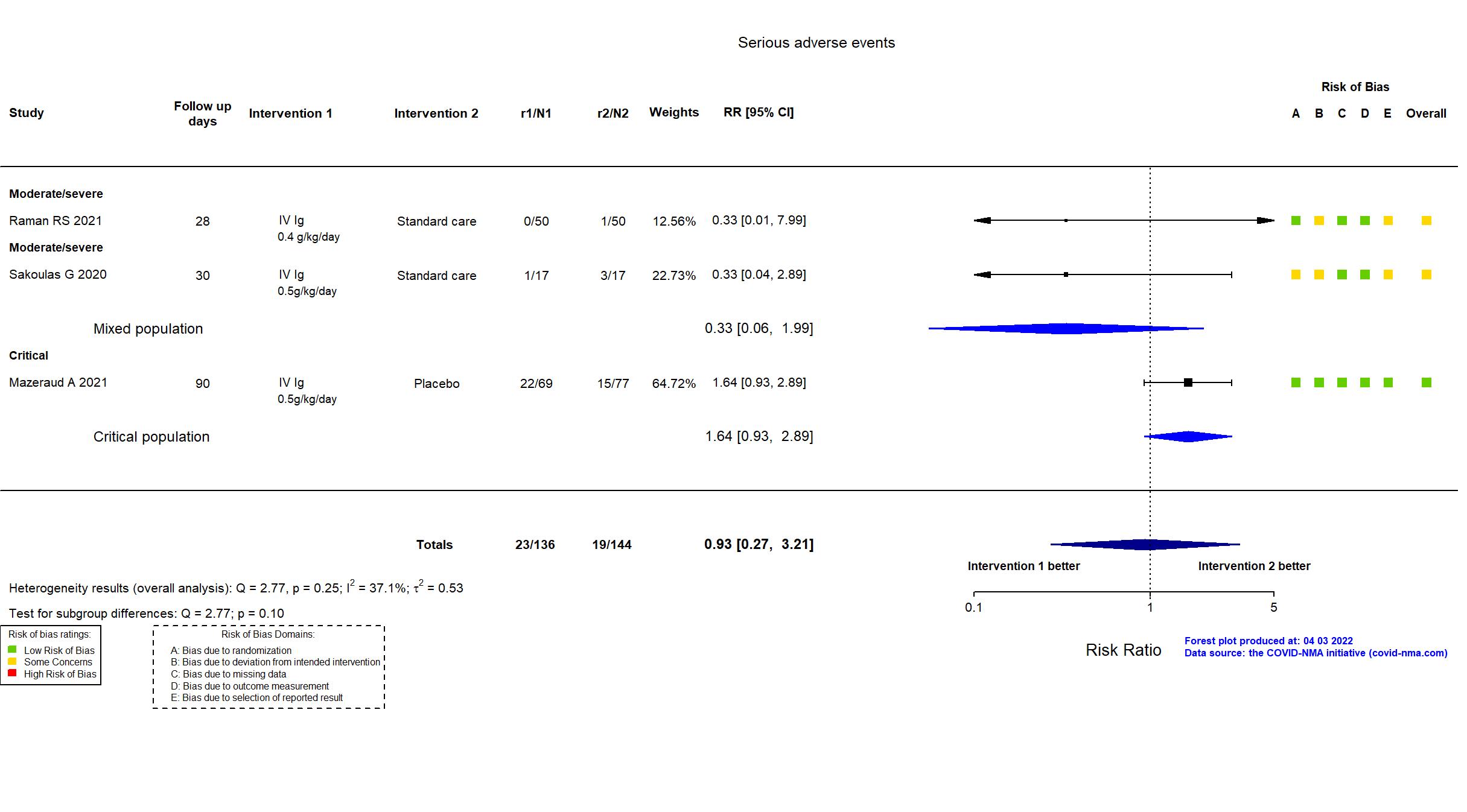
Studies included but not extracted/included in the analysis: NCT04480424 results posted on clinicaltrials.gov
FOREST PLOTS -2022-04-29










Trial IRCT20200501047259N1
Publication Gharebaghi N, BMC Infect Dis (2020) (published paper)
Dates: 2020-05-09 to 2020-06-09
Funding: Public/non profit (Urmia University of Medical Sciences)
Conflict of interest: No
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Single center / Iran Follow-up duration (days): * | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
IV Ig 5 g IV four times a day for 3 consecutive days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : IV Ig=30 Placebo=29 | |
| Characteristics of participants N= 59 Mean age : NR 41 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=* Critical: n=* | |
| Primary outcome | |
| In the register Increasing of patient's O2 saturation above 90%, improvement of lung involvement in lung CT scan | |
| In the report In-hospital mortality | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
* |
| General comment |
This study is pending author reply. The timepoints for outcomes were not reported.
In addition to the pre-print article, the study registry was used in data extraction and risk of bias assessment. The study achieved the target sample size reported in the registry. There is no change from the trial registration in the intervention and control treatments. Safety analysis was not performed. |
Trial NCT04350580; EudraCT2020-001570-30
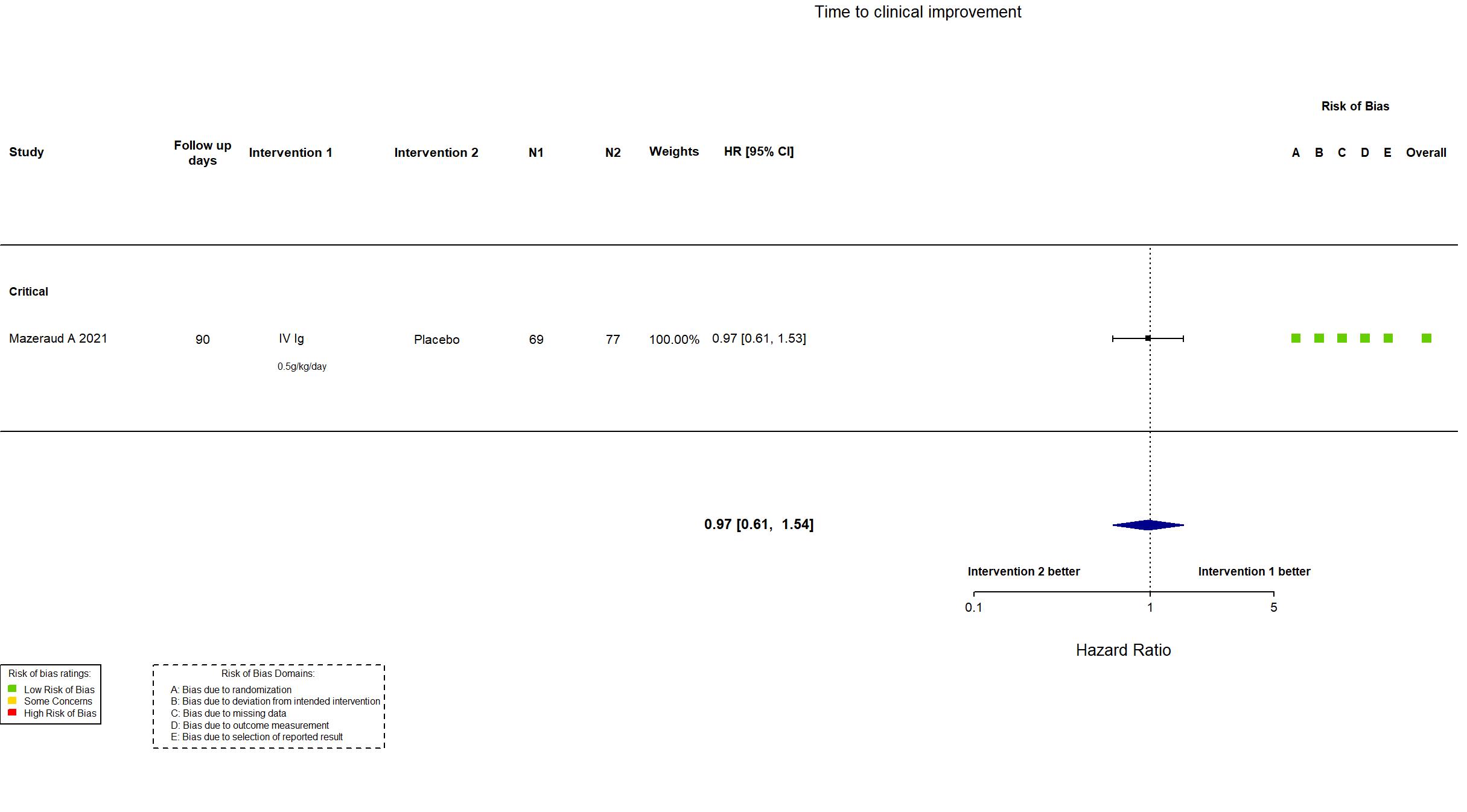
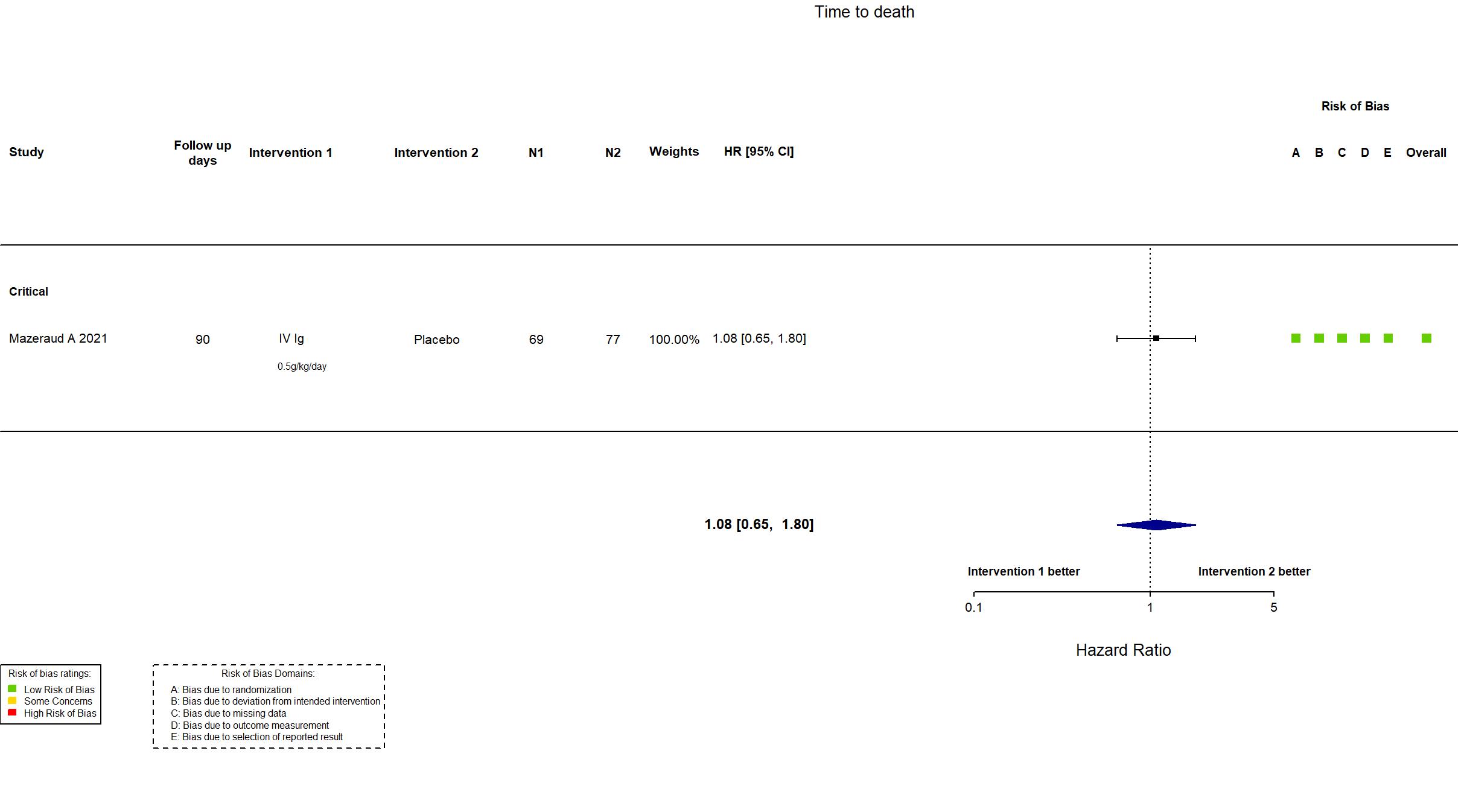
Publication ICAR - Mazeraud A, Lancet Respir Med (2021) (published paper)
Dates: 2020-04-03 to 2020-10-20
Funding: Public/non profit (Programme Hospitalier de Recherche Clinique; French ministry of Health; Laboratoire Français du fractionnement et des Biotechnologies)
Conflict of interest: No
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / France Follow-up duration (days): 90 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
IV Ig Four intravenous perfusions of 0.5g/kg each given over at least 8 h over 4 days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Placebo=77 IV Ig=69 | |
| Characteristics of participants N= 146 Mean age : NR 103 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=0 Critical: n=146 | |
| Primary outcome | |
| In the register Ventilator-free days [ Time Frame: 28 days ] | |
| In the report Number of ventilator-free days at day 28, defined as the number of days between the last extubation day and day 28. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Low |
| General comment |
In addition to the published article, the protocol, statistical analysis plan, supplementary material and study registry were used in data extraction and risk of bias assessment. The study (n=146) achieved the target sample size specified in the trial registry (n=138). There is no change from the trial registration in the intervention and control treatments. The primary outcome indicated in registry reflects the primary outcome reported in the paper.
This study was updated on March 16th, 2022 with data extracted after contact with authors. |
Trial CTRI/2020/06/026222
Publication Raman RS, J Infect Dis (2021) (published paper)
Dates: 2020-06-29 to 2020-09-30
Funding: Private (Virchow Biotech)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / India Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
IV Ig 0.4 g/kg body weight IV once daily for 5 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Standard care=50 IV Ig=50 | |
| Characteristics of participants N= 100 Mean age : NR 33 males Severity : Mild: n=0 / Moderate: n=* / Severe: n=* Critical: n=0 | |
| Primary outcome | |
| In the register Number of days to clinical improvement. It is defined as no. of days from initiation of treatment day to discharge day | |
| In the report Number of days from initiation of treatment to hospital discharge | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published accepted manuscript, the prospective study registry was used in data extraction and risk of bias assessment. Neither study protocol nor statistical analysis plan was available. The target sample size specified in the registry was achieved. There was no important change from the trial registration in the inclusion criteria or intervention and control treatments. Adverse events and serious adverse events were reported but were not pre-specified as outcomes in the registry. |
Trial NCT04411667
Publication Sakoulas G, Crit Care Expl (2020) (published paper)
Dates: 2020-05-01 to 2020-06-16
Funding: drug donation only (no specific funding, IVIG (Octagam 10%) was provided by Octapharma USA, Hoboken, NJ (donation))
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / USA Follow-up duration (days): 30 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
IV Ig 0.5 g/kg IV once a day for 3 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Standard care=17 IV Ig=17 | |
| Characteristics of participants N= 34 Mean age : NR 20 males Severity : Mild: n=0 / Moderate: n=7 / Severe: n=26 Critical: n=0 | |
| Primary outcome | |
| In the register Mechanical Ventilation [ Time Frame: from date of patient admission to date of patient discharge or date of death, whichever came first, assessed up to 45 days ] | |
| In the report Respiratory failure requiring receipt of mechanical ventilation (a composite of either receiving ventilation or the subject status changed to a do not resuscitate/do not intubate resulting in progressive respiratory failure and death) | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to all available versions of the published article, the study registry was used in data extraction. The protocol or and statistical analysis plan were available. There is no change from the trial registration in the intervention and control treatments. Quote: "For subjects not already receiving glucocorticoid therapy, enrolled treatment arm subjects received methylprednisolone 40 mg IV once 30 minutes before IVIG to mitigate headache commonly experienced after IVIG therapy." Quote: "Immediately after randomization and notification of the principal investigator, one subject was immediately deemed unevaluable by the principal investigator and excluded due to a high risk of bacterial superinfection (elevated absolute neutrophil count of 9900/mm3 and concomitant procalcitonin of 1.45 ng/mL). On August 25th, 2020 we received additional information from authors on this study. |
Trial IRCT20151227025726N20
Publication Tabarsi P, Int Immunopharmacol (2020) (published paper)
Funding: Public/non profit (Shahid Beheshti University of Medical Sciences)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / Iran Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
IV Ig 400 mg/kg IV once a day for 3 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Standard care=32 IV Ig=52 | |
| Characteristics of participants N= 84 Mean age : NR 65 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=84 Critical: n=0 | |
| Primary outcome | |
| In the register Need for mechanical ventilation, need of admission to critical care unit, and death. | |
| In the report Need for invasive mechanical ventilation and oxygenation, the need for admission to the Intensive Care Unit (ICU), and the mortality rate. | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the study registry was used in data extraction and risk of bias assessment. The study protocol and statistical analysis plan were not available at time of extraction. The target sample size specified in the registry was achieved. Some outcomes that were reported (e.g., length of hospital stay, length of ICU stay, lab values) were not pre-specified in the registry. There is no change from the trial registration in the intervention and control treatments. All participants in the intervention group only were pre-medicated with hydrocortisone, paracetamol, and antihistamine 30 minutes before the IV Ig injection. |