Therapeutic anticoagulant vs Prophylactic anticoagulant (RCT)
Hospitalized patients
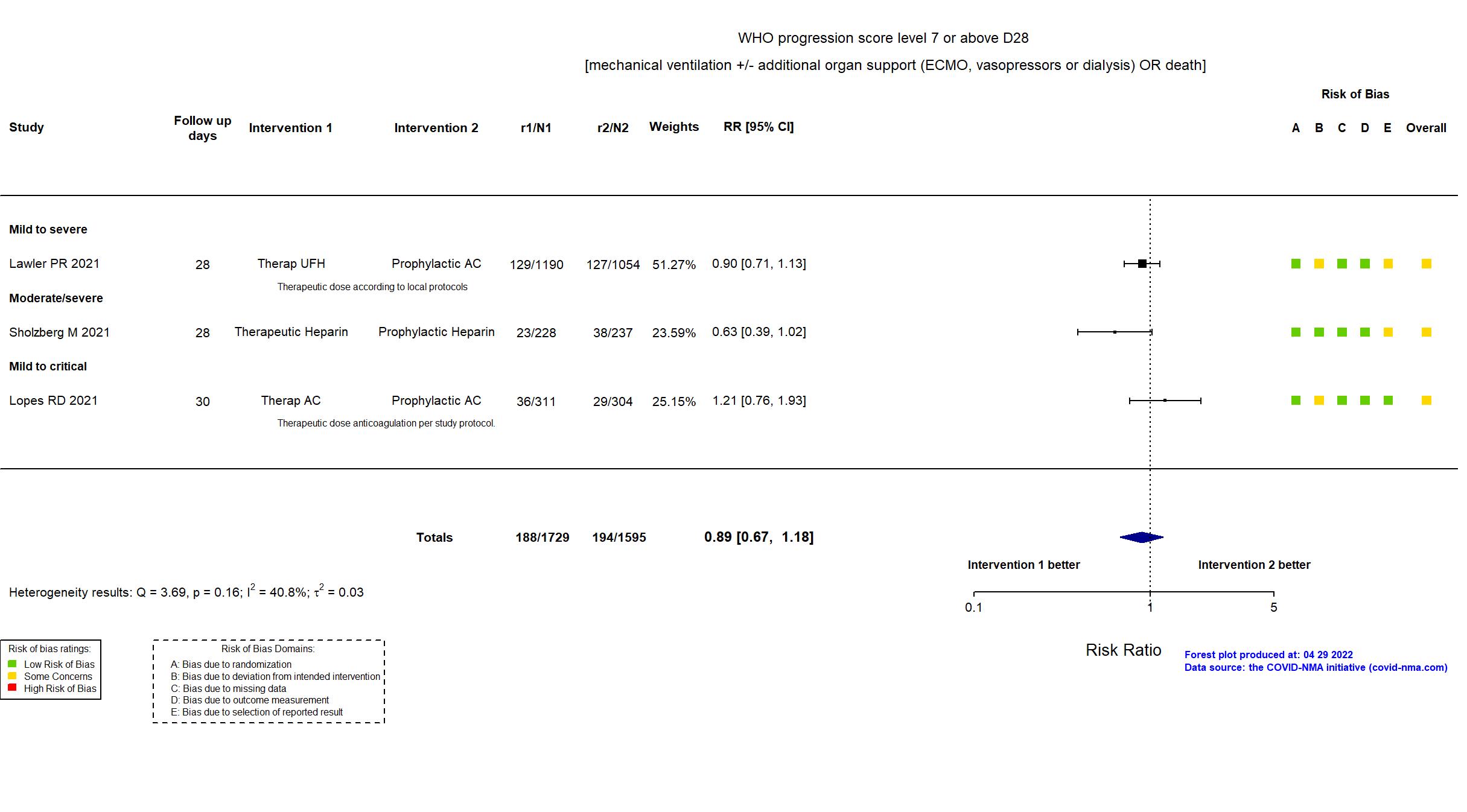
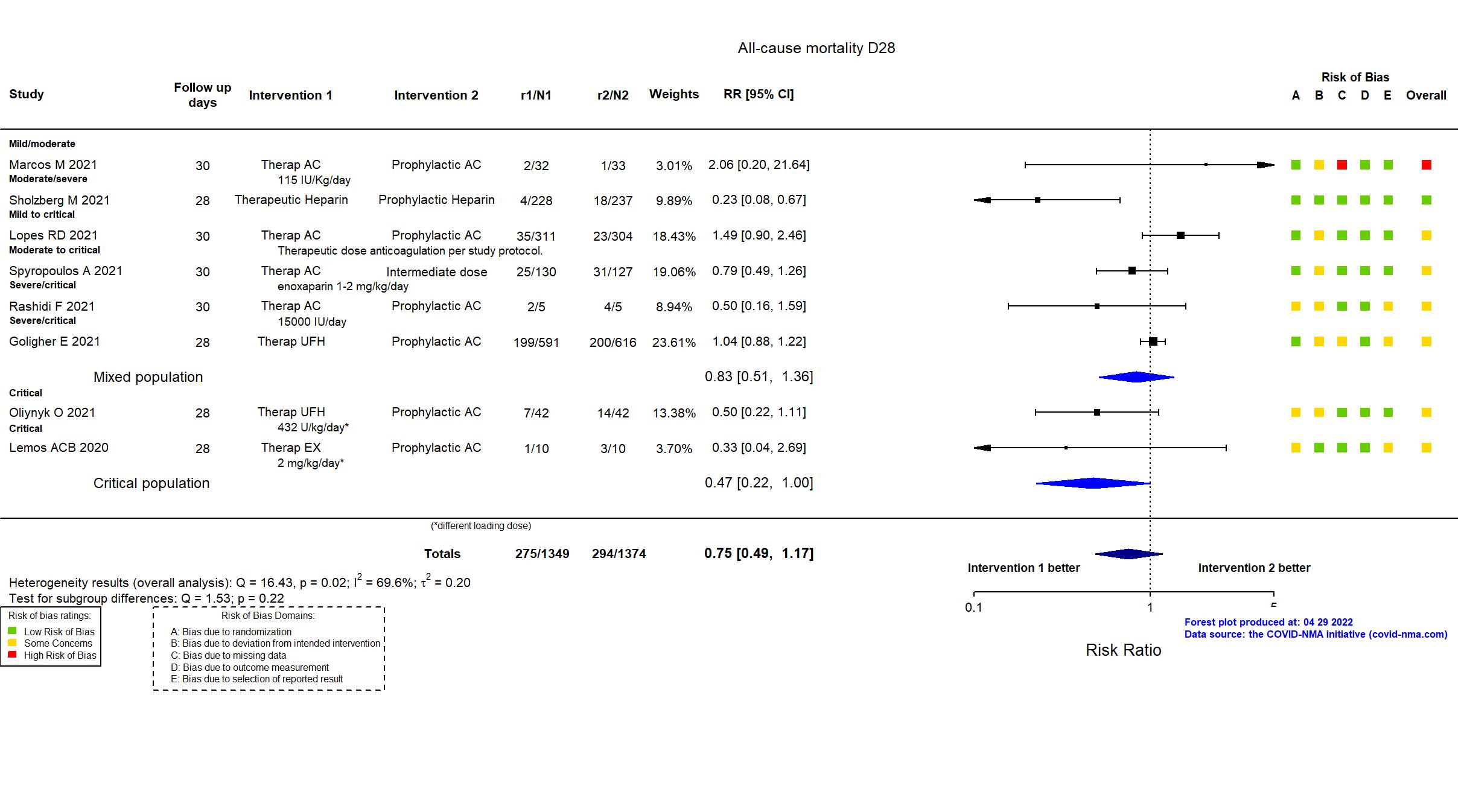
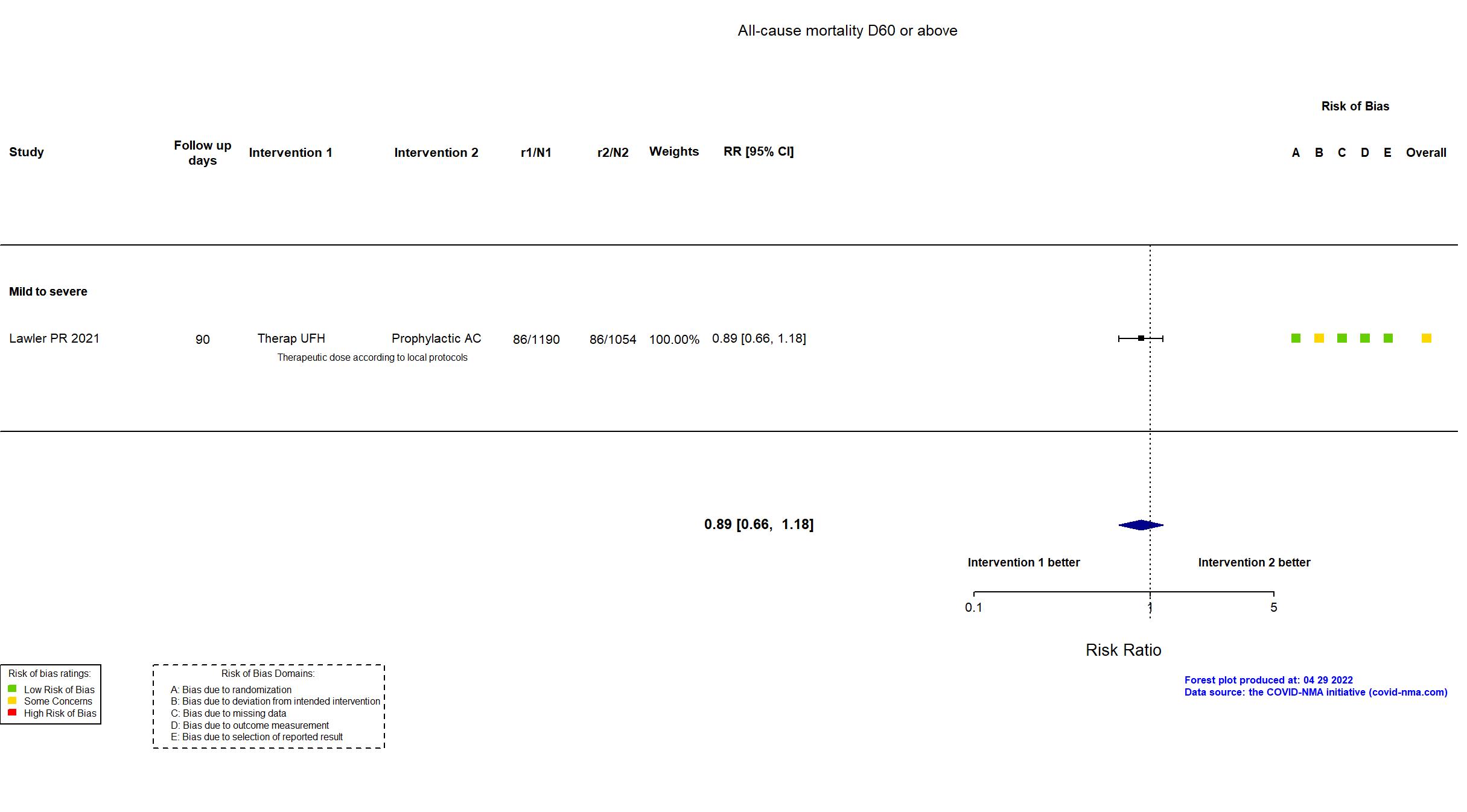
FOREST PLOTS -2022-04-29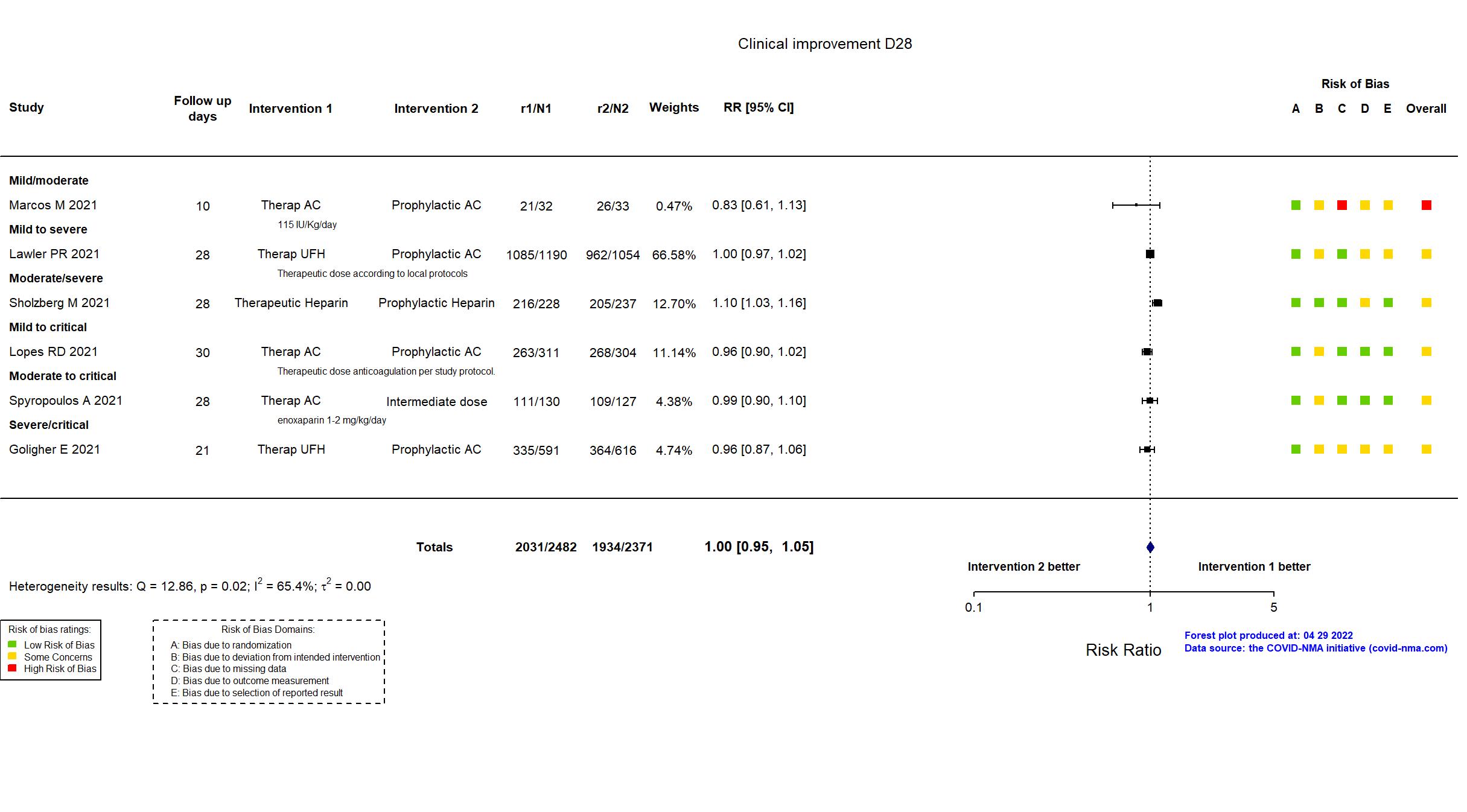








Trial NCT02735707, NCT04505774, NCT04359277, NCT04372589
Publication Goligher E, N Engl J Med (2021) (published paper)
Dates: 2020-04-21 to 2020-12-19
Funding: Mixed (Multiple funders, internationally, with multiple regional sponsors)
Conflict of interest: *
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Australia, Brazil, Canada, Ireland, Mexico, Netherlands, New Zealand, Saudi Arabia, UK, USA Follow-up duration (days): 90 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Therap UFH Therapeutic anticoagulation according to local practice (IV unfractionated heparin or SC low molecular weight heparin) for up to 14 days or until hospital discharge or liberation from the need for supplemental oxygen, whichever comes first For UFH, suggested target for aPTT of 1.5 to 2.5 times the upper limit of normal or therapeutic anti-Xa levels Low molecular weight heparin dosed according to patient weight and creatinine clearance. |
|
| Control
Prophylactic AC Standard thromboprophylaxis according to local practice (enoxaparin, dalteparin, tinzaparin, fondaparinux, or heparin) for up to 14 days or until hospital discharge or liberation from the need for supplemental oxygen, whichever comes first | |
| Participants | |
| Randomized participants : Therap UFH=591 Prophylactic AC=616 | |
| Characteristics of participants N= 1207 Mean age : NR 772 males Severity : Mild: n=* / Moderate: n=* / Severe: n=773 Critical: n=315 | |
| Primary outcome | |
| In the register For the mpRCT, organ support-free days (OSFD). The endpoint is the number of days, out of the first 21 days after randomization, that a patient is alive and free of ICU-level organ support. | |
| In the report Organ support-free days (OSFDs), an ordinal scale composed of survival to hospital discharge and, in survivors, the number of days free of organ support to day 21 | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published/pre-print article, the study registries, protocol, and statistical analysis plan were used in data extraction and risk of bias assessment. The article reports preliminary results for the severe/critical subgroup of patients in three international adaptive platform trials with harmonized protocols that evaluated the effect of an anticoagulation protocol using predominantly therapeutic dosing versus standard anticoagulation using predominantly prophylactic dosing. The three trials were REMAP-CAP (NCT02735707), ACTIV-4a (NCT04505774 and NCT04359277), and ATTACC (NCT04372589). The individual trial registries reflect each trial’s individual primary objectives, while the harmonized protocol reflects the objectives of this comparison. There were no major differences in population, procedures and intervention between the protocol and the published/pre-print article, and the outcomes reported are appropriate for a preliminary report. Recruitment of severe/critical patients was halted after interim analysis revealed futility.
On 12th of August, 2021, this study was updated based on the published report. |
Trial NCT02735707, NCT04505774, NCT04359277, NCT04372589
Publication Lawler PR, N Engl J Med (2021) (published paper)
Dates: 2020-04-21 to 2021-01-22
Funding: Mixed (Canadian Institutes of Health Research, LifeArc Foundation, Thistledown Foundation, Research Manitoba, Ontario Ministry of Health, Peter Munk Cardiac Centre, Cancercare Manitoba Foundation, Victoria General Hospital Foundation, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, National Institutes of Health (NIH), European Union, Australian National Health and Medical Research Council, Health Research Council of New Zealand, U.K. NIHR, NIHR Imperial Biomedical Research Centre, Health Research Board of Ireland, UPMC Learning While Doing Program, Breast Cancer Research Foundation, French Ministry of Health, the Minderoo Foundation, Amgen, Eisai, Global Coalition for Adaptive Research, Wellcome Trust.)
Conflict of interest: *
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Australia, Brazil, Canada, Mexico, Nepal, Netherlands, Spain, UK, USA. Follow-up duration (days): 90 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Therap UFH Therapeutic dose low molecular weight or unfractionated heparin administered according to local protocols used for the treatment of acute venous thromboembolism for up to 14 days or until recovery. |
|
| Control
Prophylactic AC Prophylactic dose low molecular weight or unfractionated heparin administered according to local practice. | |
| Participants | |
| Randomized participants : Therap UFH=1190 Prophylactic AC=1054 | |
| Characteristics of participants N= 2244 Mean age : NR 1310 males Severity : Mild: n=* / Moderate: n=* / Severe: n=98 Critical: n=0 | |
| Primary outcome | |
| In the register ATTACC/ACTIV-4 - The primary endpoint in the trial is days alive and free of organ support at day 21. This endpoint is defined as the number of days that a patient is alive and free of organ support through the first 21 days after trial entry. Organ support is defined as receipt of invasive or non-invasive mechanical ventilation, high flow nasal oxygen (>30 L/min), vasopressor therapy, or ECMO support. Death at any time (including beyond 21 days) during the index hospital stay is assigned the worst possible score of -1. REMAP-CAP - All-cause mortality [ Time Frame: Day 90 ] ; Days alive and not receiving organ support in ICU [ Time Frame: Day 21 ] | |
| In the report Organ support–free days, evaluated on an ordinal scale that combined in-hospital death and the number of days free of cardiovascular or respiratory organ support up to day 21 among patients who survived to hospital discharge | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published/pre-print article, the trial registries, protocol and statistical plan for the multi-platform RCT and supplementary appendices were used in data extraction and assessment of risk of bias. The study reports results from three adaptive randomized controlled trial protocols evaluating therapeutic-dose anticoagulation with heparin versus standard care anticoagulation in patients hospitalized for Covid-19 that were integrated into a single multi-platform RCT. Other than minor differences between the three merged studies, there were no major differences between the combined protocol and trial registries in populations, procedures, interventions or outcomes. Mortality was reported as in hospital mortality by day 90, while time to death was based on survival until hospital discharge. The trial was stopped when prespecified criteria for superiority were met for therapeutic-dose anticoagulation.
On 12th of August, 2021, this study was updated based on the published report. |
Trial RBR-949z6v
Publication Lemos ACB, Thromb Res (2020) (published paper)
Dates: 2020-04-01 to 2020-07-30
Funding: No specific funding (None)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / Brazil Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Therap EX 1 mg/kg subcutaneously twice a day (maximum 140 mg twice a day) for 4-14 days |
|
| Control
Prophylactic AC heparin: 5000 IU subcutaneously 3 times a day (if weight < 120 kg) and 7500 IU 3 times a day (if weight > 120 kg) OR enoxaparin: 40 mg once daily (if weight < 120 kg) and 40 mg twice daily (if weight > 120 Kg) according to the doctor's judgment. | |
| Participants | |
| Randomized participants : Therap EX=10 Prophylactic AC=10 | |
| Characteristics of participants N= 20 Mean age : NR 16 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=0 Critical: n=20 | |
| Primary outcome | |
| In the register Evaluation of gas exchange between D0 / D4 /D7 /D14 evaluated through the PO2 / FIO2 ratio; days without mechanical ventilation (within 28 days of follow-up, how many days were out of mechanical ventilation, if the patient dies before 28 days of foll | |
| In the report Variation in gas exchange over time evaluated through the ratio of partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2) at baseline, 7, and 14 days after randomization | |
| Documents avalaible |
Protocol Yes. In language other than English Statistical plan NR Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published study report, the protocol (available in Portuguese) and trial registry were used in data extraction and assessment of risk of bias. The study differed from its original protocol in that there are only two arms in the reported trial whereas three were intended, but the authors are transparent about this, stating the reason for the abandonment of the third arm. The reported study achieved the sample size proposed in the protocol. Whereas the protocol stated that Enoxaparin may be extended up to 7-10 days at the discretion of the medical team, the published article reports maintaining treatment for up to 14 days. There were no substantive differences in outcomes between the registry or protocol and the published study report. |
Trial NCT04394377
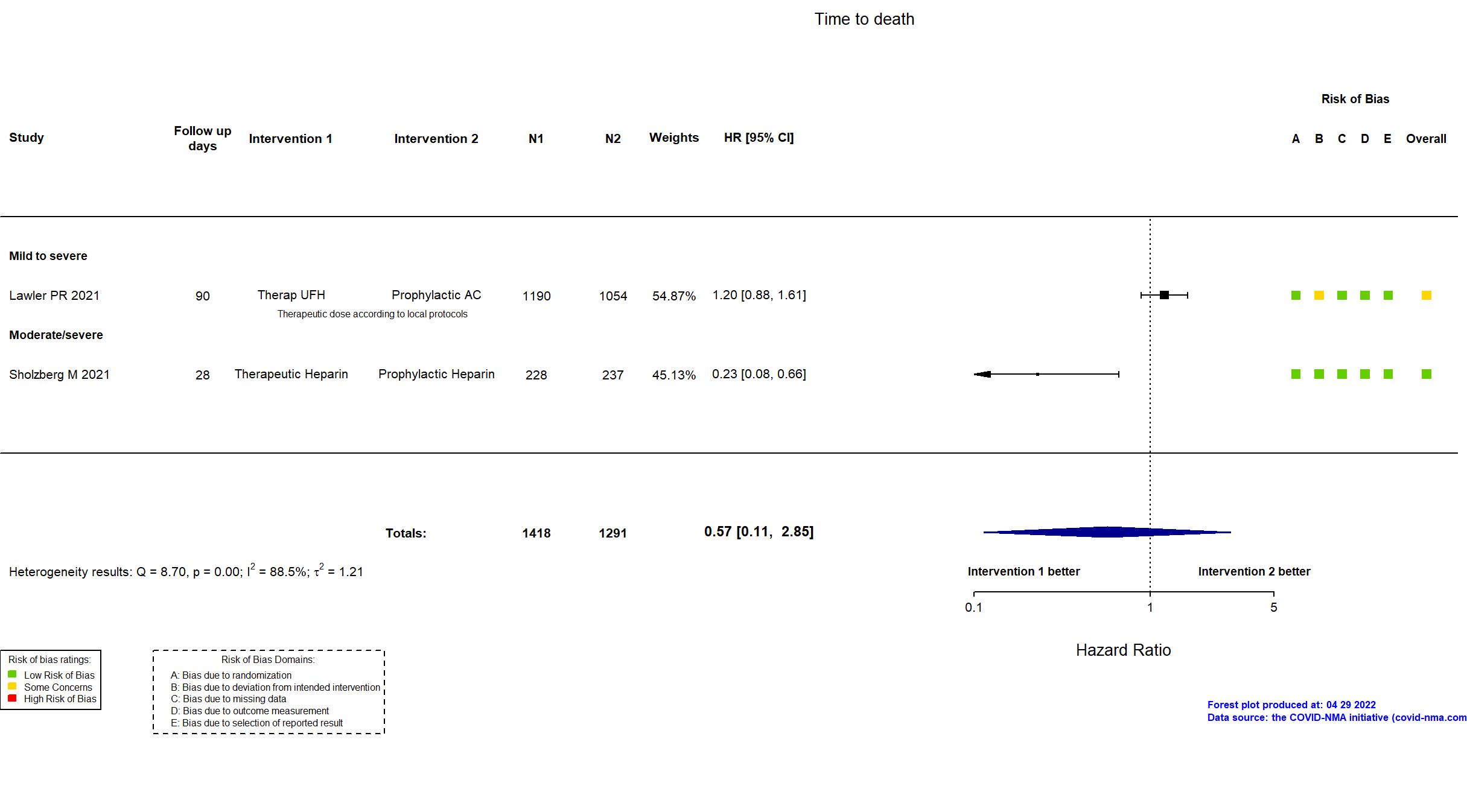
Publication ACTION - Lopes RD, Lancet (2021) (published paper)
Dates: 2020-06-24 to 2021-02-26
Funding: Mixed (Coalition COVID-19 Brazil, Bayer SA.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Brazil Follow-up duration (days): 60 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Therap AC Clinically stable patients: PO rivaroxaban, 20 mg once daily (15 mg once daily if reduced creatinine clearance). Clinically unstable patients: SC enoxaparin 1 mg/kg twice per day, or IV unfractionated heparin at a dose to achieve anti-Xa concentration or partial thromboplastin time targets. Unfractionated heparin preferred option for patients with renal dysfunction or disseminated intravascular coagulation. Treatment to day 30. |
|
| Control
Prophylactic AC Standard IV thromboembolism prophylaxis with enoxaparin or unfractionated heparin during hospitalisation and extended at the discretion of the treating physician. | |
| Participants | |
| Randomized participants : Therap AC=311 Prophylactic AC=304 | |
| Characteristics of participants N= 615 Mean age : NR 368 males Severity : Mild: n=155 / Moderate: n=369 / Severe: n=53 Critical: n=38 | |
| Primary outcome | |
| In the register Hierarchical composite endpoint composed of mortality, number of days alive, number of days in the hospital and number of days with oxygen therapy at the end of 30 days. [ Time Frame: In 30 days ] | |
| In the report Hierarchical composite of time to death, duration of hospitalisation, or duration of supplemental oxygen use through 30 days; Major or clinically relevant non-major bleeding | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the registry, published and unpublished protocols and statistical analysis plan, and supplementary appendices were used in data extraction and assessment of risk of bias. The WHO 8-point ordinal scale (on which data extracted were based) was not in the registry or first version of the study protocol, but was added in an approved amendment to the protocol within 2 weeks of start of recruitment. There were no differences between the protocol and the published article in populations, procedures or interventions. The study achieved its pre-specified target sample size. |
Trial NCT04604327
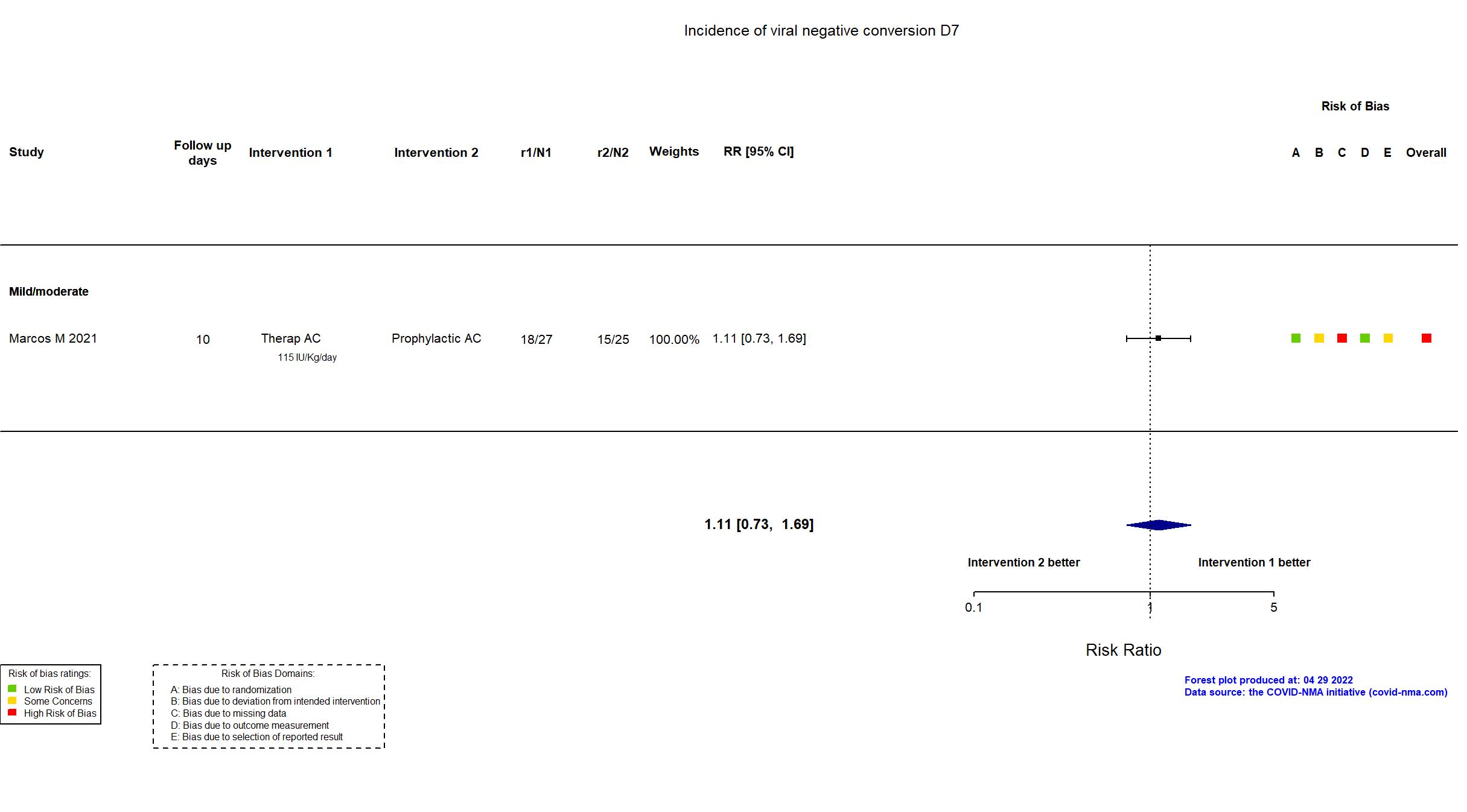
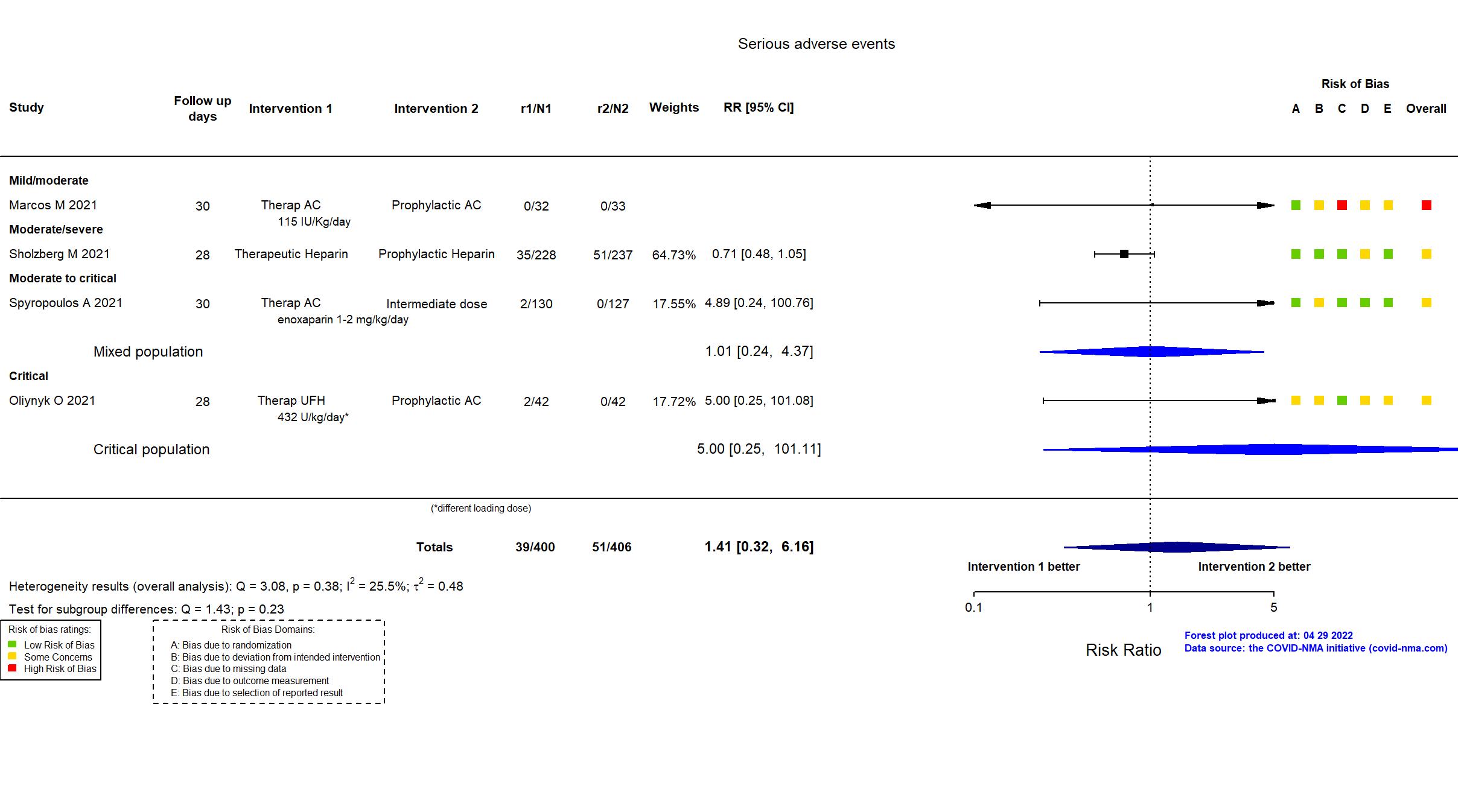
Publication Marcos M, Thromb Haemost (2021) (published paper)
Dates: 2020-10-26 to 2021-05-30
Funding: Mixed (This trial was an investigator-initiated study. Laboratorios Farmacéuticos ROVI kindly provided the study drugs, but did not participate in any other step of the clinical trial process.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Spain Follow-up duration (days): 30 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Therap AC 115 IU/Kg subcutaneously, adjusted to body weight (7,500 IU for patients between 50-70 Kg; 10,000 IU for patients weighing >70-100 Kg; 12,500 IU for patients who weighed >100 Kg) once a day for 10 days (extended at the discretion of the treating physician) |
|
| Control
Prophylactic AC 3,500 IU subcutaneously once a day for 10 days (extended at the discretion of the treating physician) | |
| Participants | |
| Randomized NR Analyzed 65 participants Prophylactic AC=33 Therap AC=32 | |
| Characteristics of participants N= 65 Mean age : NR 41 males Severity : Mild: n=27 / Moderate: n=38 / Severe: n=0 Critical: n=0 | |
| Primary outcome | |
| In the register Clinical deterioration [ Time Frame: 10 days ] Combined outcome that includes number of patients who suffer any of the following: Death, ICU admission, mechanical ventilatory support, progression to moderate or severe ARDS (according to Berlin criteria) or arterial or venous thrombosis. | |
| In the report Composite of death, ICU admission, need of mechanical ventilation support, development of moderate/severe acute respiratory distress syndrome and venous or arterial thrombosis within 10 days of enrollment | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
High |
| General comment | In addition to the published article, the study registry was used in data extraction and risk of bias assessment. Neither protocol nor statistical analysis plan was available. There is no change from the trial registration in the intervention and control treatments. he primary outcome in the article reflects the primary outcome in the registry. The secondary outcomes in the article were not included in the registry. Recruitment to the study was terminated after an interim analysis, conducted at 40% of target sample size, because of both slow recruitment and futility. As a result the trial did not achieve its target sample size (164 versus 65). |
Trial 0112U001413 (Ukraine)
Publication Oliynyk O, Life (2021) (published paper)
Dates: 2020-07-01 to 2021-03-01
Funding: Public/non profit (Ministry of Science and Higher Education in Poland)
Conflict of interest: No
| Methods | |
| RCT | |
| Location :
Single center / Ukraine Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Therap LMWH 100 IU/kg/day subcutaneously twice a day until D-dimer normalized Therap UFH Initial dose: 80 U/kg/h intravenously; Maintenance dose: 18 U/kg/h intravenously until the D-dimer values normalized |
|
| Control
Prophylactic AC 50 IU/kg/day subcutaneously once a day until D-dimer normalized | |
| Participants | |
| Randomized participants : Prophylactic AC=42 Therap LMWH=42 Therap UFH=42 | |
| Characteristics of participants N= 126 Mean age : NR 76 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=0 Critical: n=126 | |
| Primary outcome | |
| In the register NR | |
| In the report NR | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | Only the published article was used in data extraction and risk of bias assessment. The protocol, statistical analysis plan and study registry were not available. There is no information on whether the study achieved the target sample size or if the interventions, control treatments or outcomes were determined a-priori. Of note, 4 patients in the UFH (control) group were withdrawn from the study due to adverse events and provided with LMWH (treatment), but there was no information on which LMWH treatment arm they were moved to or which group they were analyzed in. |
Trial *
Publication Rashidi F, Thromb Res (2021) (published paper)
Dates: 2020-09-01 to 2021-04-30
Funding: Not reported/unclear
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Iran Follow-up duration (days): 30 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
tPA Initial dose: 25 mg infused over 2 hr, 25 mg for the next 22 h - followed by UFH until aPTT of 50–70/sec until hospital discharge Therap AC 5000 IU of UFH subcutaneously three times a day until hospital discharge |
|
| Control
Prophylactic AC Initial dose: 80 IU/kg UFH - maintenance dose: 18 mg/kg by continuous infusion until aPTT of 50–70/sec until hospital discharge | |
| Participants | |
| Randomized participants : tPA=5 Therap AC=5 Prophylactic AC=5 | |
| Characteristics of participants N= 15 Mean age : NR 11 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=5 Critical: n=10 | |
| Primary outcome | |
| In the register NR | |
| In the report P/F ratio improvement during the first 48-h of enrollment | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | Only the published article was used in data extraction and assessment of risk of bias. No registry, protocol or statistical analysis plan was available and so it is not clear whether the primary outcome reported reflected a pre-specified outcome or whether the study achieved a target sample size. |
Trial NCT04362085; NCT04444700
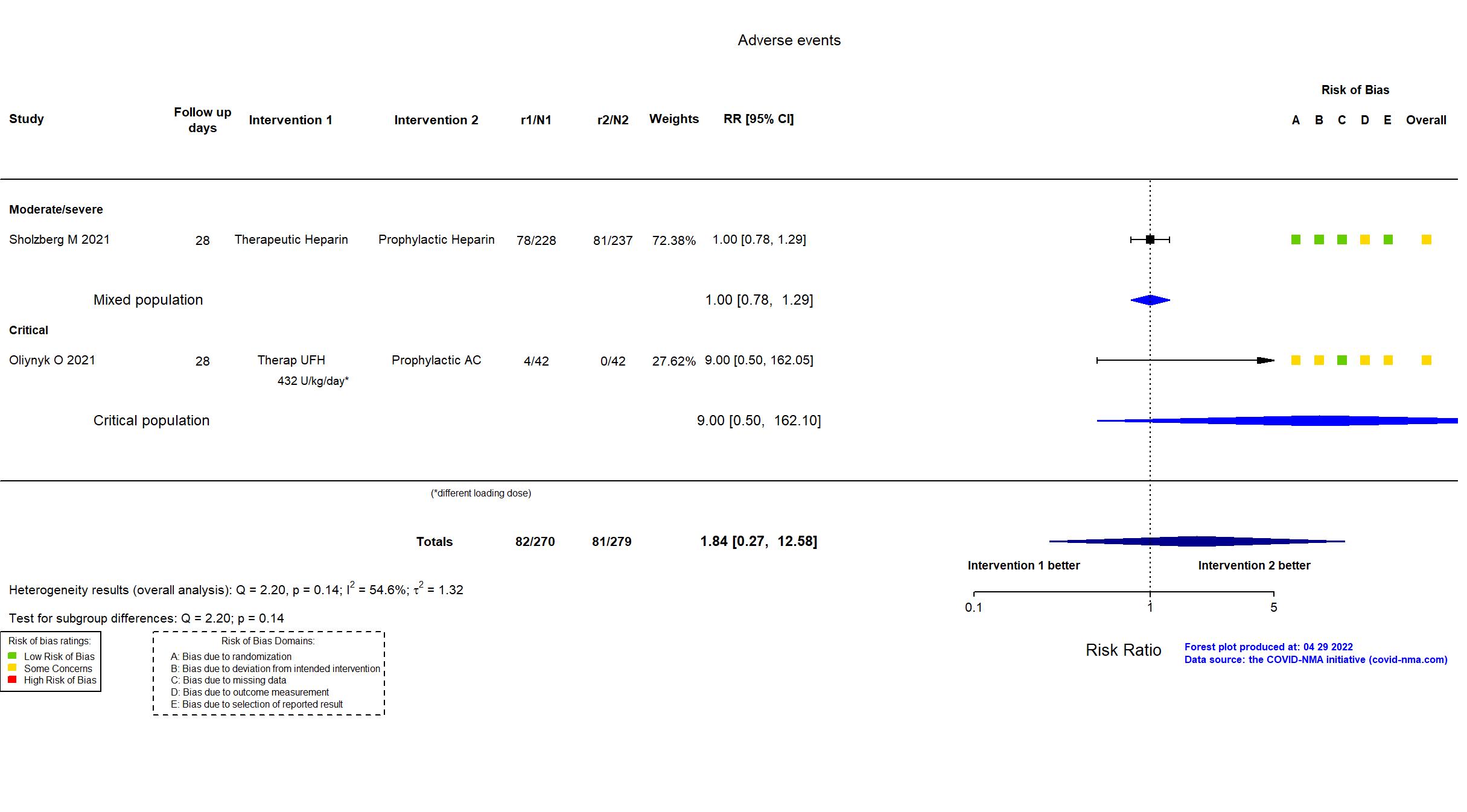
Publication RAPID - Sholzberg M, BMJ (2021) (published paper)
Dates: 2020-05-29 to 2021-04-12
Funding: Public/non profit (Task 54, Department of National Defence; St. Michael’s Hospital Foundation; St. Joseph’s Health Centre Foundation; 2020 TD Community Health Solutions Fund – COVID-19 Research Grant; Michael Garron Hospital; The Ottawa Hospital Foundation COVID-19 Emergency Response Fund; INVENT Kickstarter Award; Science Foundation Ireland, Enterprise Ireland, IDA Ireland COVID-19 Rapid Response Funding; SEAMO COVID-19 Innovation Fund; National Institute of General Medical Sciences, NIH; University of Vermont Medical Center Fund Grant; King Saud University)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Brazil, Canada, Ireland, Saudi Arabia, UAE, USA Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Therapeutic Heparin Creatinine clearance ≥30 + BMI <40: - Enoxaparin 1 mg/kg SC every 12 hours OR 1.5 SC mg/kg every 24 hours; - Dalteparin 200 units/kg SC every 24 hours OR 100 IU/kg SC every 12 hours; - Tinzaparin 75 U/kg SC every 24 hours; - unfractionated heparin IV bolus, with continuous infusion to titrate to institution specific anti-Xa or aPTT values. Creatinine clearance ≥30 + BMI ≥40: - Enoxaparin 1 mg/kg every 12 hours; - Dalteparin 100 units/kg SC every 12 hours; - Tinzaparin 175 U/kg SC daily; - unfractionated heparin IV bolus, with continuous infusion to titrate to institution specific anti-Xa or aPTT values. Creatinine clearance <30: - UFH IV bolus, with continuous infusion to titrate to institution specific anti-Xa or aPTT values or LMWH per hospital protocol. Administered until hospital discharge, death, day 28 or study withdrawal. |
|
| Control
Prophylactic Heparin Creatinine clearance ≥30 + BMI <40: - Enoxaparin 40 mg SC every 24 hours; - Dalteparin 5000 units SC q24h; - Tinzaparin 4500 U SC every 24 hours; - Fondaparinux 2.5 mg SC every 24 hours; - Unfractionated Heparin 5000 units SC every 8-12 hours. Creatinine clearance ≥30 + BMI ≥40: - Enoxaparin 40 mg SC every 24 hours; - Dalteparin 5000 units SC every 12 hours; - Tinzaparin 9000 (+/- 1000) U SC every 24 hours; - Unfractionated Heparin 7000 units SC every 8 hours. Creatinine clearance <30 + BMI <40: - UFH 5000 units SC every 8-12 hours or LMWH per hospital protocol. Creatinine clearance <30 + BMI ≥40: - UFH 7500 units SC every 8 hours or LMWH per hospital protocol. Administered until hospital discharge, death, day 28 or study withdrawal. | |
| Participants | |
| Randomized participants : Therapeutic Heparin=228 Prophylactic Heparin=237 | |
| Characteristics of participants N= 465 Mean age : NR 264 males Severity : Mild: n=* / Moderate: n=* / Severe: n=* Critical: n=0 | |
| Primary outcome | |
| In the register Composite outcome of ICU admission (yes/no), non-invasive positive pressure ventilation (yes/no), invasive mechanical ventilation (yes/no), or all-cause death (yes/no) up to 28 days. [ Time Frame: up to 28 days ] | |
| In the report Composite of ICU admission, non-invasive (bilevel or continuous positive airway pressure) or invasive mechanical ventilation, or death up to 28 days | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published article, the pre-print, study registries, protocol, supplementary appendix and data gained from contact with authors were used in data extraction and risk of bias assessment. There is no change from the trial registration in the intervention and control treatments. The registry primary outcome reflects the reported primary outcome.
Quote: "RAPID had an adaptive design. The protocol prespecified that the sample size would be increased if the conditional power at 75% of the original sample size was between 60 and 80%.21 However, the conditional power was below 60%, therefore the sample size was not increased, thus RAPID remained underpowered." The study was updated on the November 24th, 2021 after the published article. The study was updated on the April 25th, 2022 after contact with the authors. |
Trial NCT04401293
Publication Spyropoulos A, JAMA Intern Med (2021) (published paper)
Dates: 2020-05-08 to 2021-05-14
Funding: Public/non profit (Feinstein Institutes for Medical Research, the Broxmeyer Fellowship in Clinical Thrombosis, and grant R24AG064191 from the National Institute on Aging)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: single blinding | |
| Location :
Multicenter / USA Follow-up duration (days): 30 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Therap AC 1 mg/kg subcutaneously twice daily if CrCl was 30 mL/min/1.73 m2 or greater or 0.5 mg/kg twice daily if CrCl was 15-29 mL/min/1.73 m2. Postdischarge anticoagulation allowed at the discretion of treating physicians. |
|
| Control
Intermediate dose Unfractionated heparin ≤ 22 500 IU subcutaneously daily; enoxaparin, 30 mg or 40 mg subcutaneously once or twice daily; dalteparin 2500 IU or 5000 IU subcutaneously daily. If CrCl fell below 15 mL/min/1.73 m2, enoxaparin was converted to treatment-dose intravenous UFH until CrCl >15 mL/min/1.73 m2. Postdischarge anticoagulation allowed at the discretion of treating physicians. | |
| Participants | |
| Randomized participants : Therap AC=130 Intermediate dose=127 | |
| Characteristics of participants N= 257 Mean age : NR 136 males Severity : Mild: n=* / Moderate: n=192 / Severe: n=39 Critical: n=13 | |
| Primary outcome | |
| In the register Composite outcome of arterial thromboembolic events, venous thromboembolic events and all-cause mortality at Day 30 ± 2 days. [ Time Frame: Day 30 ± 2 days ] Risk of arterial thromboembolic events (including myocardial infarction, stroke, systemic embolism), venous thromboembolism (including symptomatic deep vein thrombosis (DVT) of the upper or lower extremity, asymptomatic proximal DVT of the lower extremity, non-fatal pulmonary embolism (PE)), and all-cause mortality at Day 30 ± 2 days. | |
| In the report VTE (symptomatic upper or lower extremity deep vein thrombosis, asymptomatic lower extremity proximal deep vein thrombosis, symptomatic pulmonary embolism, splanchnic vein thrombosis, or cerebral sinus thrombosis), or ATE (myocardial infarction, ischemic stroke, peripheral or systemic ATE) or death from any cause within 30 ± 2 days after randomization | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: N |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published article, the registry, published and full protocols with statistical analysis plan and supplementary appendices as well as data from contact with authors were used in data extraction and assessment of risk of bias. The primary and secondary outcomes in the article reflected those in the registry. The trial (n = 257) achieved its target sample size (n = 246). There is no change from the trial registration in the intervention and control treatments.
The study was updated on the March 28th, 2022 with data from authors. |