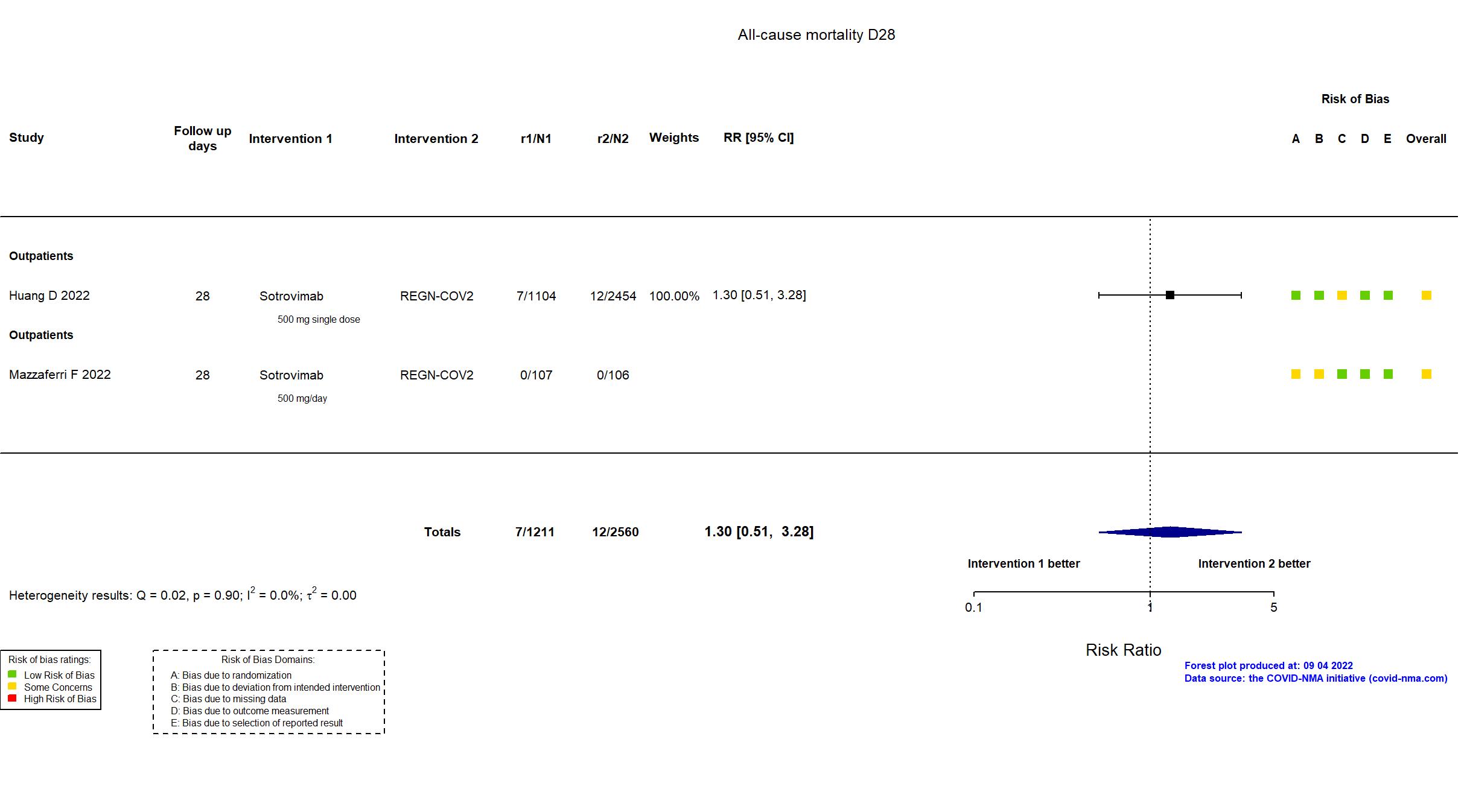
Sotrovimab vs Casirivimab+Imdevimab (REGN-COV2) (RCT)
Mild outpatients
FOREST PLOTS -2023-01-27



Trial NCT04790786
Publication OPTIMISE-C19 - Huang D, JAMA Netw Open (2022) (published paper)
Funding: Mixed (The Federal COVID Response Team (formerly called Operation Warp Speed) supplies mABs to UPMC, participates in design discussions, and is provided regular updates. UPMC supports this trial with resources and internal funds, and leadership was involved in all aspects of the trial including design, analysis, data interpretation, and manuscript preparation. The US government provided casirivimab-imdevimab, and GlaxoSmithKline and Vir Biotechnology provided sotrovimab. Preliminary results were shared with GlaxoSmithKline and Vir Biotechnology before preprint submission, but the organization had no role in manuscript preparation or interpretation of results.)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / USA Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Sotrovimab 500 mg Intravenously single dose |
|
| Control
REGN-COV2 2400 mg (1200 mg of each drug) intravenously single dose | |
| Participants | |
| Randomized NR Analyzed 3558 participants Sotrovimab=1104 REGN-COV2=2454 | |
| Characteristics of participants N= 3558 Mean age : NR 1639 males Severity : Mild: n= 3558/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register Alive and Free from Hospitalization [Time Frame: 28 days after initial participation]: Days alive and free from hospitalization. Patients that are both living and not in the hospital will meet criteria to be counted in this outcome. | |
| In the report Hospital-free days up to day 28 after mAb treatment. This outcome was an ordinal end point, with death as the worst outcome (labeled as −1) followed by the length of time alive and free of hospitalization, such that the best outcome would be 28 hospital-free days. If a patient had intervening days free of hospitalization and was then rehospitalized, the patient was given credit for the intervening days as free of hospitalization. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the trial registry, protocol, statistical analysis plan and supplementary appendices were used in data extraction and assessment of risk of bias. There is no change from the trial registration in the intervention and control treatments. The primary outcome in the article reflects that in the registry. Some outcomes from the registry are not reported in the paper (e.g., mortality at 90 days, viral negative conversion). Authors noted, "With a pragmatic adaptive design, this trial had no set sample size; the sample size was dependent on case volume, mAb treatment capacity, and meeting predetermined statistical thresholds for equivalence or inferiority." The article reports an early analysis to September 2021 conducted in the context of the Delta crisis. NCT reports much higher recruitment with end of study in June 2022. |
Trial NCT05205759
Publication MANTICO - Mazzaferri F, eLife (2022) (published paper)
Dates: 2021-12-09 to 2022-01-20
Funding: Public/non profit (Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA); the ORCHESTRA (Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic) project, which has received funding from the European Union’s Horizon 2020 research and innovation programme)
Conflict of interest: No
| Methods | |
| RCT Blinding: single blinding | |
| Location :
Multicenter / Italy Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Sotrovimab 500 mg of sotrovimab by intravenous infusion single dose Bamlanivimab+Etesevimab 700 mg of bamlanivimab + 1400 mg of etesevimab by intravenous infusion single dose |
|
| Control
REGN-COV2 600 mg of casirivimab + 600 mg of imdevimab by intravenous infusion single dose | |
| Participants | |
| Randomized participants : REGN-COV2=106 Sotrovimab=107 Bamlanivimab+Etesevimab=106 | |
| Characteristics of participants N= 319 Mean age : NR 170 males Severity : Mild: n= 311/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register COVID-19 progression [ Time Frame: 14 days ] (1) hospitalization or (2) need of supplemental oxygen therapy at home or (3) death within 14 days of randomisation | |
| In the report COVID-19 progression, defined as hospitalisation, need of supplemental oxygen therapy, or death from any cause through day 14. | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published article, the pre-print article, the trial registry was used in data extraction and assessment of risk of bias. There is no change from the trial registration in the intervention and control treatments. The primary outcome in the article reflects that in the registry. Results are reported per variant of concerns (delta and omicron). Adverse events are not reported. The trial (n = 319) did not achieve its target sample size (n = 1260) as recruitment was interrupted after the publication of in-vitro evidence that two treatments under investigation (bamlanivimab/etesevimab and casirivimab/imdevimab) were not effective against the new emerging viral Omicron VOC.
This study was updated on January 19th, 2023 with data extracted from the published report. |