Ensitrelvir vs Placebo (RCT)
Mild outpatients
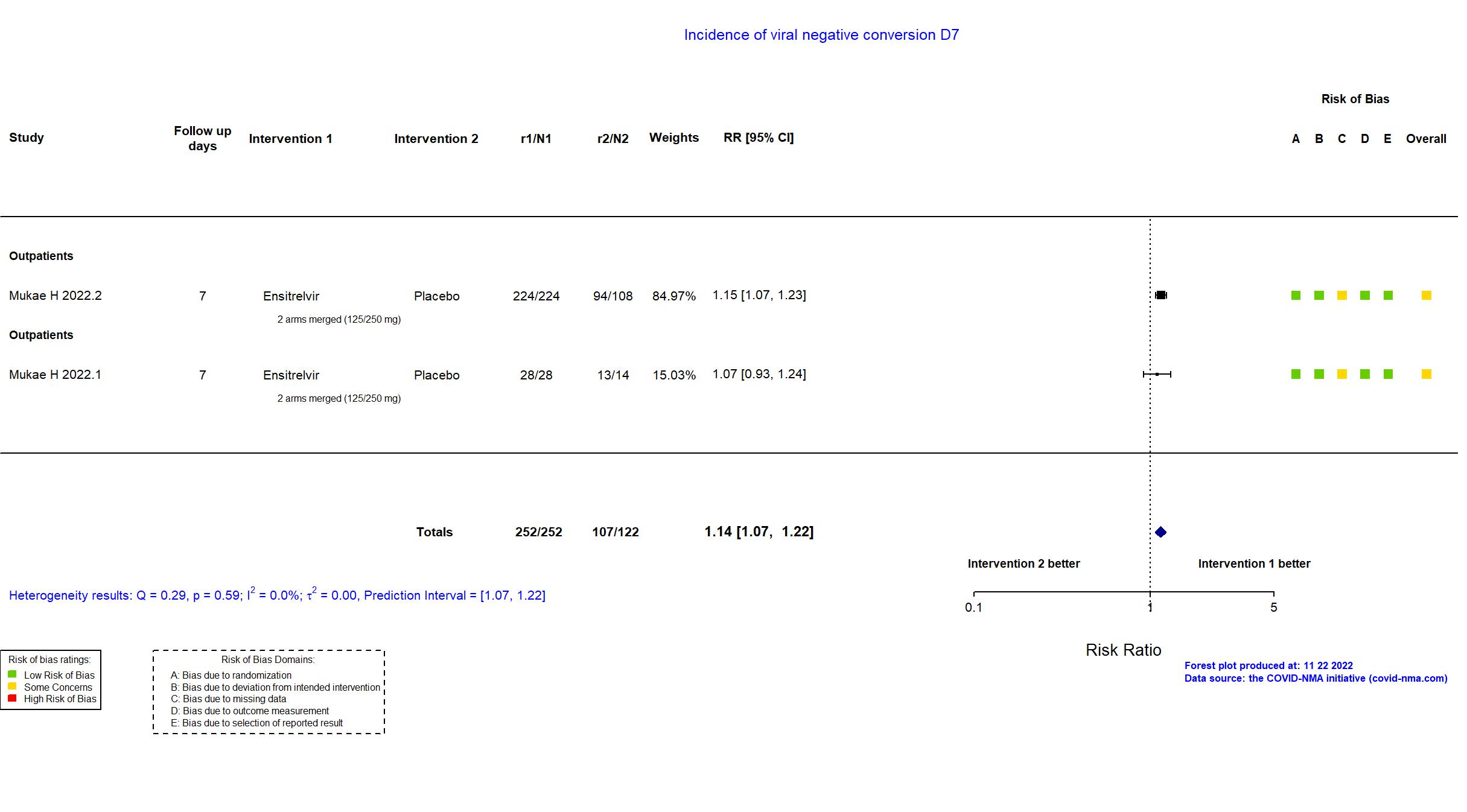
FOREST PLOTS -2022-11-22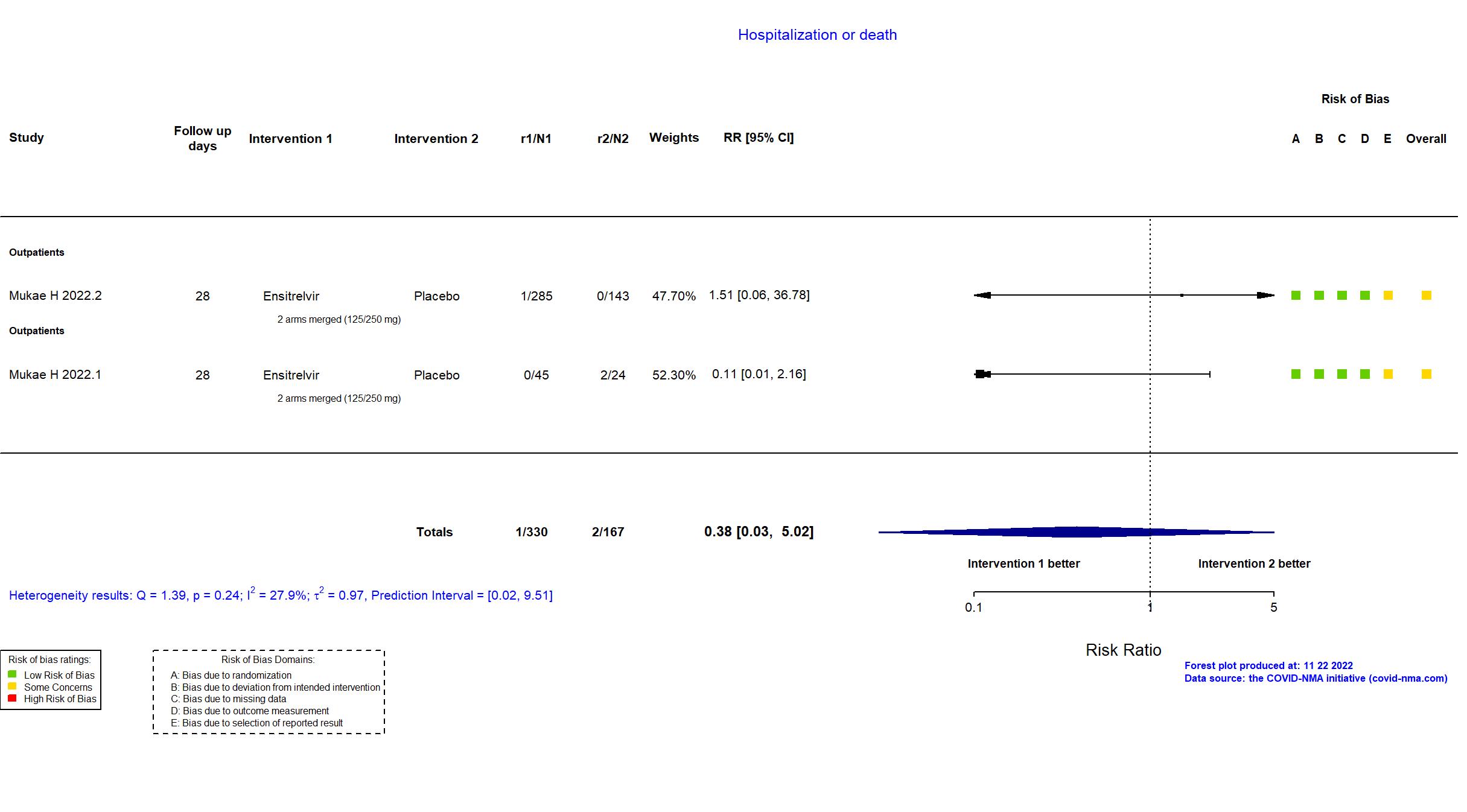
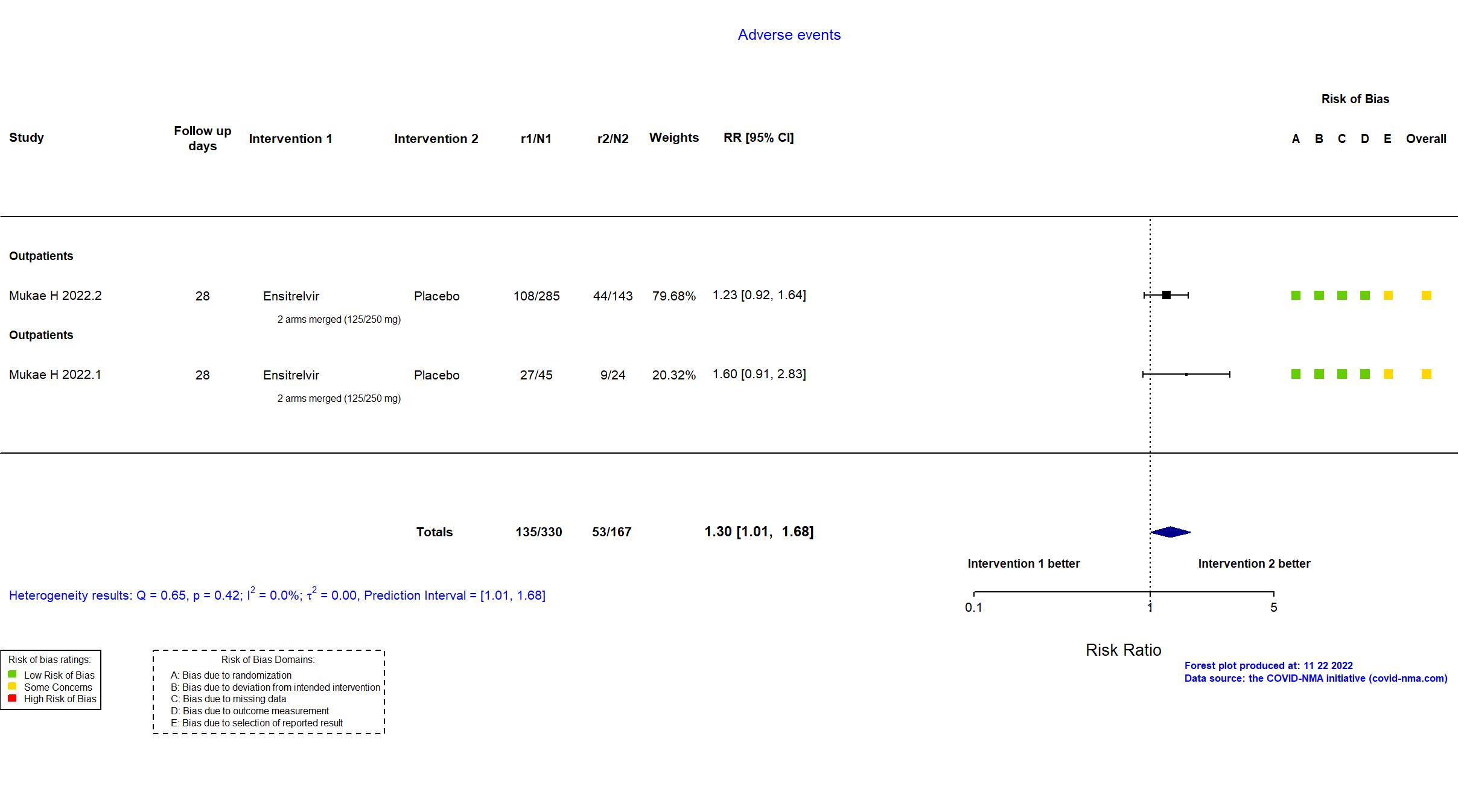
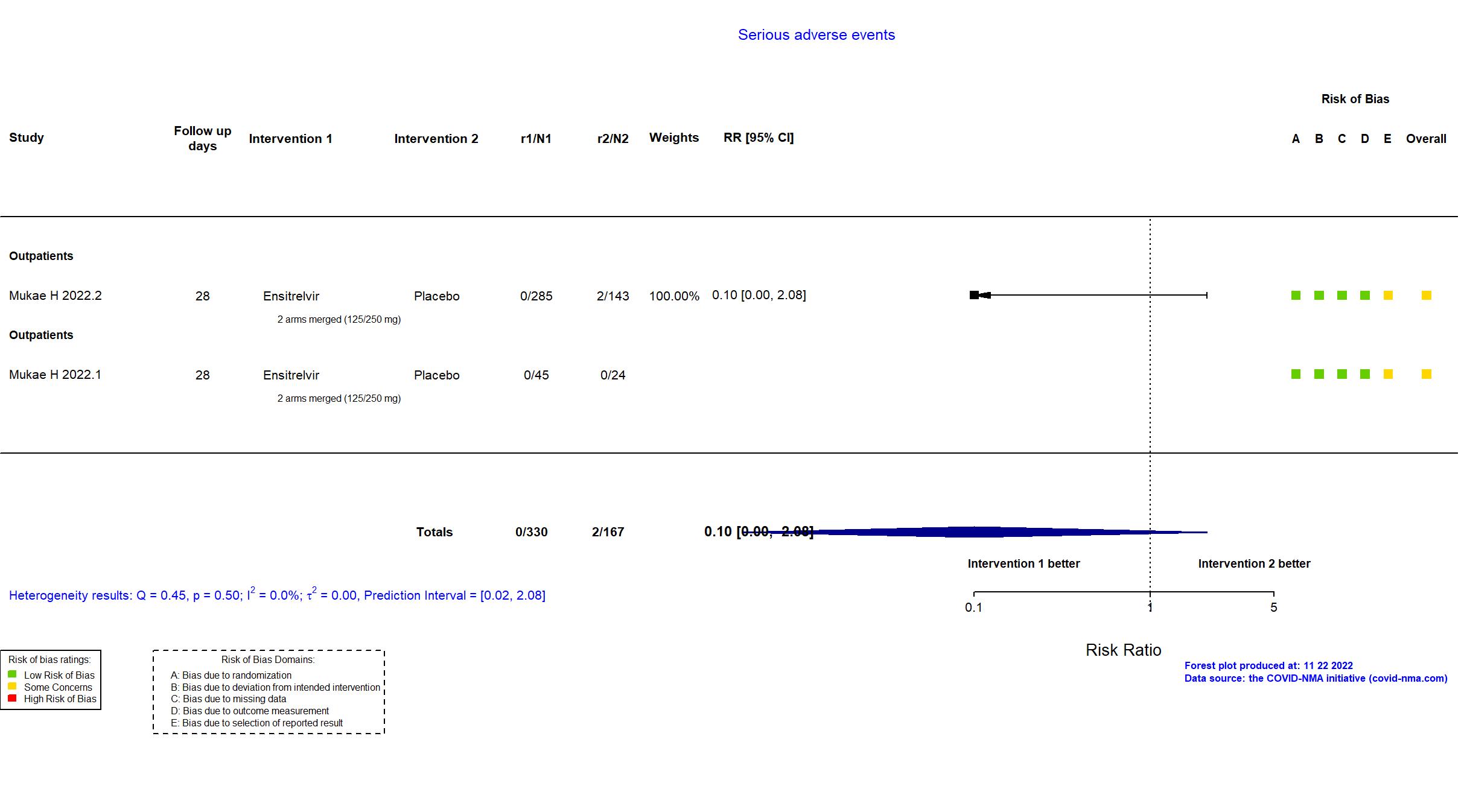
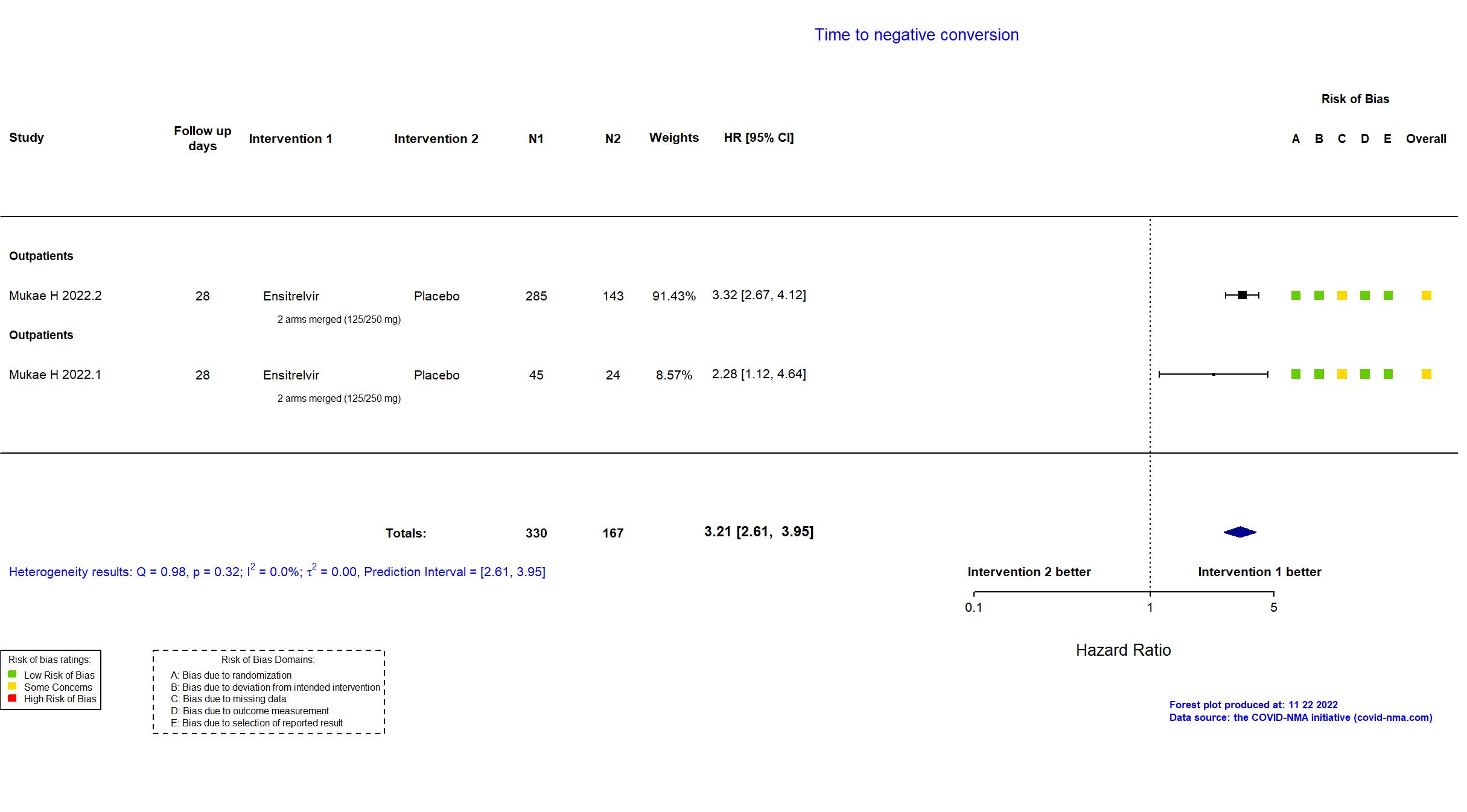







Trial jRCT2031210350
Publication Mukae H, Antimicrob Agents Chemother (2022) (published paper)
Dates: 2021-09-28 to 2022-01-30
Funding: Mixed (Shionogi & Co., Ltd ; Japan Agency for Medical Research and Development. Employees of Shionogi & Co., Ltd. participated in and approved the design and conduct of the study, wrote the protocol, and were involved in the collection, management, analysis, and interpretation of data. Institutional authors reviewed and approved the protocol and collected and interpreted the data.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: triple blinding | |
| Location :
Multicenter / Japan Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Ensitrelvir Initial dose: 375 mg orally on day 1 - Maintenance dose: 125 mg orally on days 2-5. Ensitrelvir 250 Initial dose: 750 mg orally on day 1 - Maintenance dose: 250 mg orally on days 2-5. Ensitrelvir 125 Initial dose: 375 mg orally on day 1 - Maintenance dose: 125 mg orally on days 2-5. |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Ensitrelvir=45 Ensitrelvir 250=23 Placebo=24 Ensitrelvir 125=22 | |
| Characteristics of participants N= 114 Mean age : NR 37 males Severity : Mild: n= 54/ Asymptomatic: n=9 | |
| Primary outcome | |
| In the register Change in virus titer of SARS-CoV-2 from baseline at each time point | |
| In the report Change from baseline (day 1, before drug administration) in SARS-CoV-2 viral titer on days 2, 4, 6, 9, 14, and 21 (or study discontinuation). | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the pre-print article, the trial registry and supplementary appendices were used in data extraction and assessment of risk of bias. There is no change from the trial registration in the intervention and control treatments. The primary outcome in the article reflect those in the registry. The trial (n = 69) its target sample size (n = 69). This paper reports on a part a of a 2 part phase 2 trial.
This study was updated on November 18th, 2022 with data extracted from the published report and after contact with authors. |
Trial jRCT2031210350
Publication Mukae H, medRxiv (2022) (preprint)
Dates: 2022-01-02 to 2022-02-09
Funding: Mixed (Shionogi & Co., Ltd. ; Organization of the Ministry of Health, Labour and Welfare. Employees of Shionogi & Co., Ltd., participated in and approved the design and conduct of the study; wrote the protocol; and were involved in the collection, management, analysis, and interpretation of data. Institutional authors reviewed and approved the protocol and collected and interpreted the data.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: triple blinding | |
| Location :
Multicenter / Japan, South Korea Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Ensitrelvir Initial dose: 375 mg orally once daily on day 1 - Maintenance dose: 125 mg orally once daily on days 2-5 Ensitrelvir 250 Initial dose: 750 mg orally once daily on day 1 - Maintenance dose: 250 mg orally once daily on days 2-5 Ensitrelvir 125 Initial dose: 375 mg orally once daily on day 1 - Maintenance dose: 125 mg orally once daily on days 2-5 |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Ensitrelvir=285 Placebo=143 Ensitrelvir 250=143 Ensitrelvir 125=142 | |
| Characteristics of participants N= 713 Mean age : NR 260 males Severity : Mild: n= 570/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register Change in total score of 12 symptoms of COVID-19 from Day 1 to Day 6 per unit time ; Day 4 Change in virus titer of SARS-CoV-2 from baseline. | |
| In the report Change from baseline (day 1, before drug administration) in the SARS-CoV-2 viral titer on day 4 of treatment ; change from baseline up to 120 hours in the total score of COVID-19 symptoms. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the pre-print article, the prospective trial registry and supplementary appendices were used in data extraction and assessment of risk of bias. There is no change from the trial registration in the intervention and control treatments. The primary outcomes in the article reflect those in the registry. The trial (n = 428) did not achieve its target sample size (n = 435). This paper reports on a part b of a 2 part phase 2 trial.
The study was updated on November 18th, 2022 with data extracted after contact with authors. |