Nirmatrelvir/ritonavir vs Placebo (RCT)
Mild outpatients
FOREST PLOTS -2022-03-17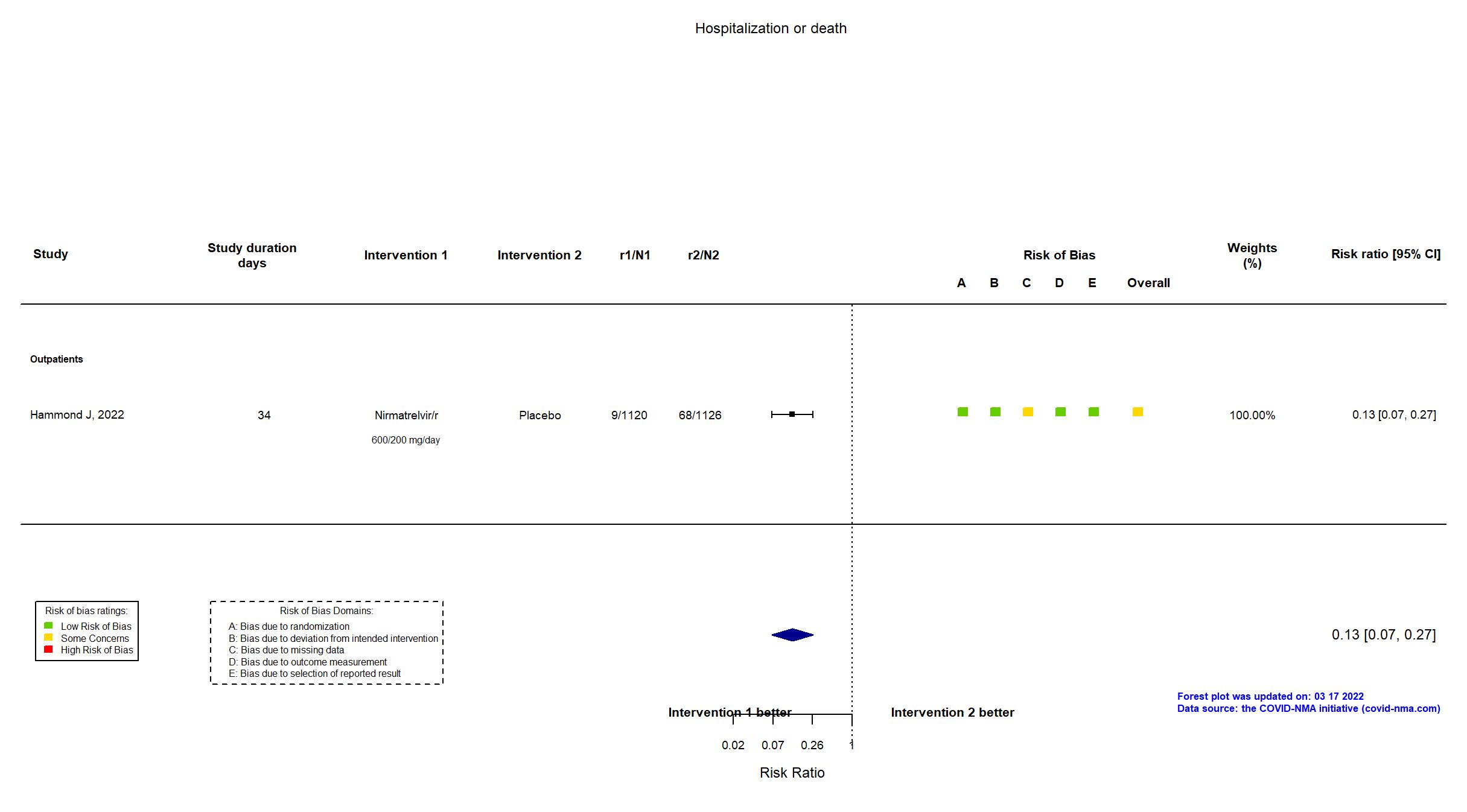




Trial NCT04960202
Publication EPIC-HR - Hammond J, N Engl J Med (2022) (published paper)
Dates: 2021-07-16 to 2021-12-09
Funding: Private (Pfizer, Inc.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / Argentina, Brazil, Bulgaria, Czech Republic, Colombia, Hungary, India, Japan, Malaysia, Mexico, Poland, Puerto Rico, Russia, Spain, South Africa, Sout Follow-up duration (days): 34 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Nirmatrelvir/r 300 mg of nirmatrelvir plus 100 mg of ritonavir orally twice daily for 5 days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Nirmatrelvir/r=1120 Placebo=1126 | |
| Characteristics of participants N= 2246 Mean age : NR 1148 males Severity : Mild: n= 2246/ Asymptomatic: n=0 | |
| Primary outcome | |
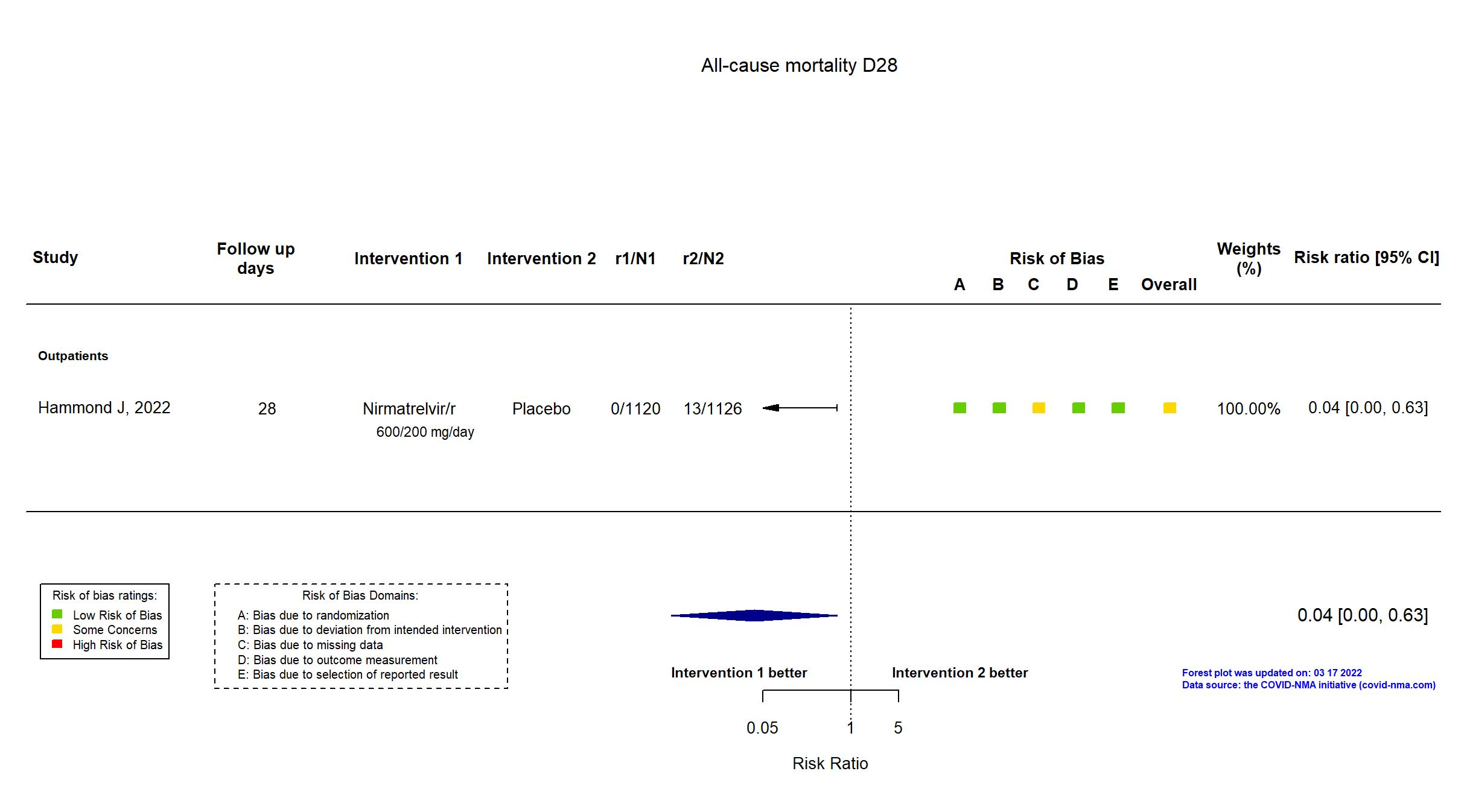
| In the register Proportion of participants with COVID-19 related hospitalization or death from any cause [Time Frame: Day 1 through Day 28] | |
| In the report Percentage of patients with Covid-19–related hospitalization or death from any cause through day 28 in the two groups | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
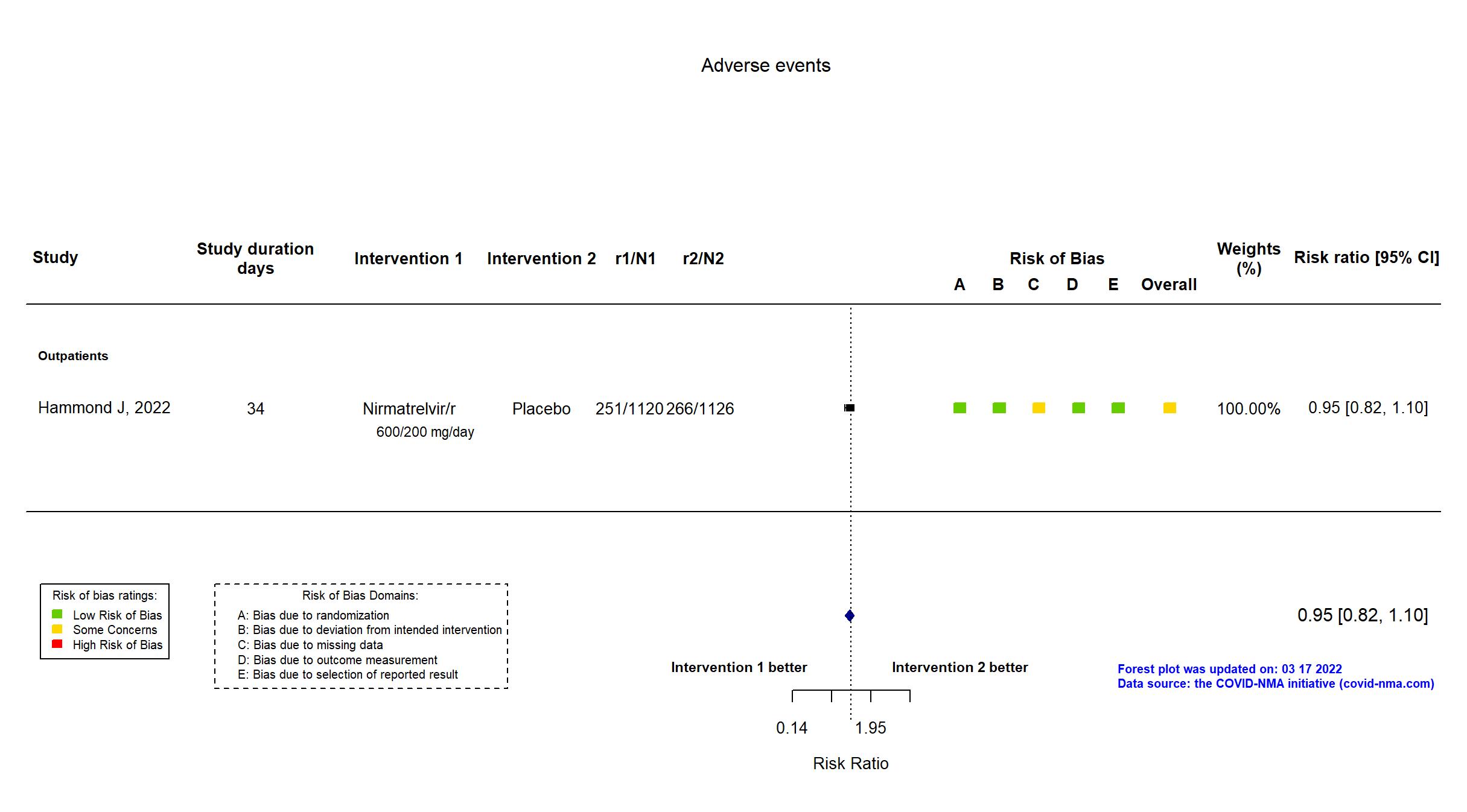
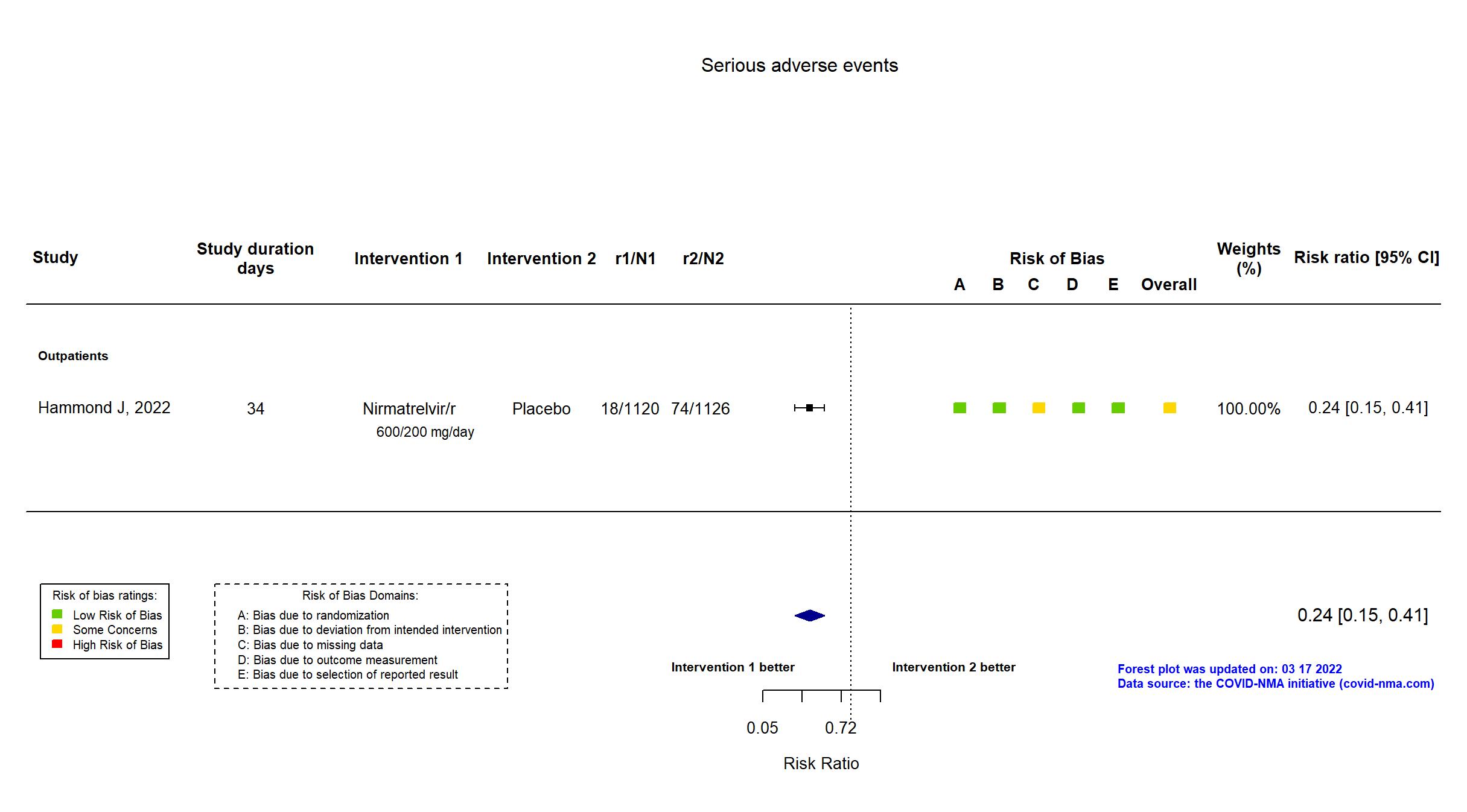
| General comment | In addition to the published article, the registry and the protocol were used in data extraction and assessment of the risk of bias. There is no change from the trial registration in the intervention and control treatments. The primary outcome in the published report reflects that in the registry and protocol, but some secondary outcomes from the registry are not reported in the paper (e.g. the number of COVID-19 related medical visits other than hospitalization and number of days in the hospital and intensive care unit for the treatment of COVID-19 [Time Frame: Day 1 through Day 34]. A planned interim analysis for efficacy and futility with a sample size-re-estimation was conducted and reviewed independently after approximately 45% of overall participants completed the Day 28 assessments in the mITT analysis set (ie, 28 days after randomization). The reason for all discontinuations was summarized by treatment group. Results of the primary outcome were extracted for the patients who were treated ≤5 days from symptom onset and regardless of mAb status in the mITT2 population. |