Vitamin C vs Standard of care/Placebo (RCT)
Mild outpatients
FOREST PLOTS -2022-04-29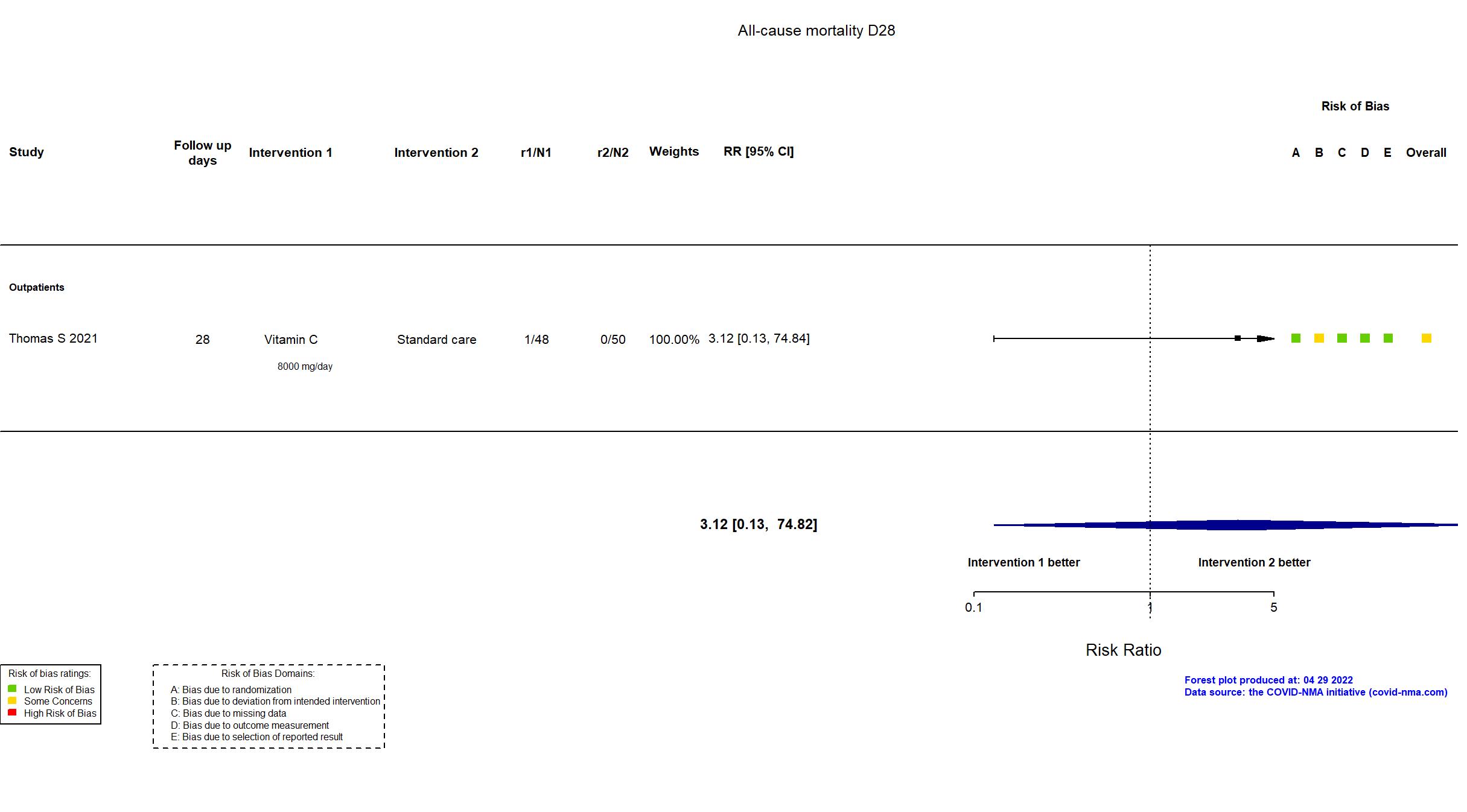



Trial NCT04342728
Publication COVID A to Z - Thomas S, JAMA Netw Open (2021) (published paper)
Dates: 2020-04-27 to 2020-10-14
Funding: Not reported/unclear (Cleveland Clinic Heart, Vascular and Thoracic Institute)
Conflict of interest: *
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / USA Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Vitamin C 8000 mg/day orally, over 2-3 times per day for 10 days Zinc 50 mg/day orally once daily for 10 days Vitamin C+Zinc Vitamin C: 8000 mg/day orally, over 2-3 times per day for 10 days Zinc: 50 mg/day orally once daily for 10 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Vitamin C=48 Zinc=58 Vitamin C+Zinc=58 Standard care=50 | |
| Characteristics of participants N= 214 Mean age : NR 82 males Severity : Mild: n= */ Asymptomatic: n=* | |
| Primary outcome | |
| In the register Symptom Reduction [ Time Frame: 28 days ] Number of days to reach a 50 percent reduction in the cumulative 0-36 symptom score with each symptom evaluated on a 0-3 scale. Assessed symptoms are Fever, Cough, Shortness of Breath, Fatigue, Muscle or body aches, Headache, New loss of taste, New loss of smell, Congestion or runny nose, Nausea, Vomiting, Diarrhea. Each patient will have a composite score ranging from 0-36/day | |
| In the report Number of days required to reach a 50% reduction in symptom severity score from peak symptom score. This end point is reported for both the 4-symptom score available for all patients and the subset of patients for whom the 12-symptom score was available. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published article, the study registry and protocol were used in data extraction and risk of bias assessment. The study was terminated early due to futility.
Quote: "Due to slower than expected enrollment, an interim analysis was conducted at approximately 40% of expected enrollment (214 of 520 patients). Stopping for superiority would only be considered if any treatment group achieved P < .001 compared with placebo. Stopping for futility would be considered if the conditional power was less than 30% for any (or all) treatment groups compared with placebo...The OSMB met on October 23, 2020, and recommended stopping the study for futility. The futility criteria was met for the 3 active treatment groups compared with the usual care group." As a result, the target sample size specified in the registry was not achieved. Mortality was reported in the paper, but was not pre-specified in the registry. Other efficacy outcomes were not reported in the paper. Quote: "In the original analysis plan, patients recorded a questionnaire with only 4 symptoms (ie, fever/chills, shortness of breath, cough, and fatigue) for scores ranging from 0 to 12. However, based on CDC guidelines, the study protocol was amended on July 16, 2020, and the symptom questionnaire was expanded to include a total of 12 symptoms (ie, fevers/chills, shortness of breath, cough, fatigue, muscle or body aches, headache, new loss of taste, new loss of smell, congestion or runny nose, nausea, vomiting, and diarrhea), creating a score ranging from 0 to 36.2 The original 4-symptom scale was collected from all patients. The 12-symptom scale was only collected from patients enrolled after the July 16, 2020, amendment." There is no change from the trial registration in the intervention and control treatments. |