Vitamin C vs Standard of care/Placebo (RCT)
Hospitalized patients
FOREST PLOTS -2021-05-27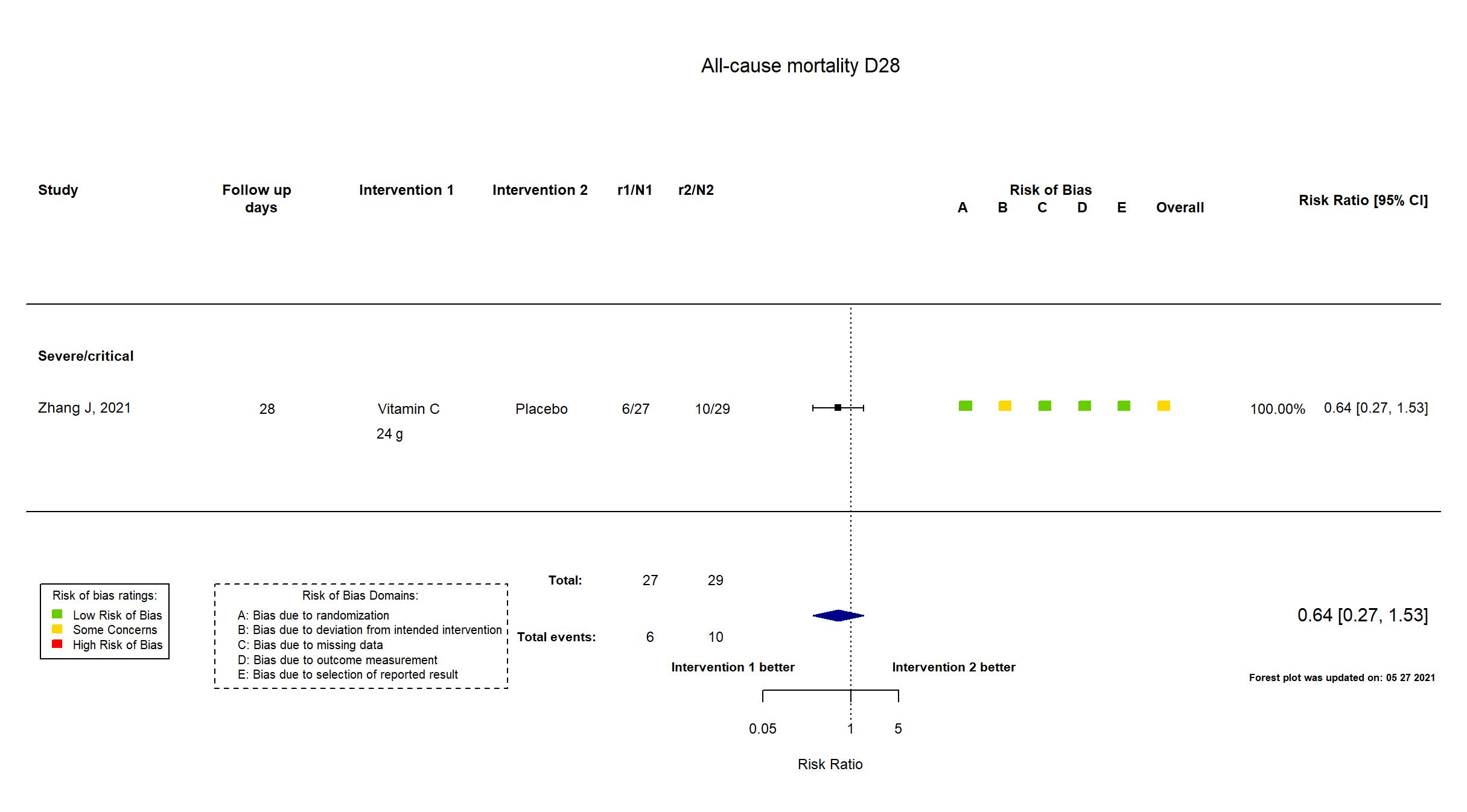


Trial IRCT20200411047025N1
Publication JamaliMoghadamSiahkali S, Eur J Med Res (2021) (published paper)
Dates: 2020-04-01 to 2020-05-30
Funding: Public/non profit (Tehran University of Medical Sciences)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / Iran Follow-up duration (days): * | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Vitamin C 1.5 g IV four times daily for 5 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Vitamin C=30 Standard care=30 | |
| Characteristics of participants N= 60 Mean age : NR 30 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=60 Critical: n=0 | |
| Primary outcome | |
| In the register improvement of SPO2 (stands for peripheral capillary oxygen saturation); daily, until 3-5 days or discharge | |
| In the report The primary endpoints in this trial were a decrease in mortality, duration of hospitalization, and need for ICU admission | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: No |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
* |
| General comment |
This study is pending contact with authors due to lack of information on outcome timepoints, and overall follow-up.
In addition to the pre-print article, the trial registry was used in data extraction and risk of bias assessment. Neither study protocol nor statistical analysis plan was available. There were some differences between the trial registry and the pre-print article in terms of population, with the inclusion criteria in the pre-print article limiting recruitment to those with more severe COVID and respiratory distress. Several outcomes reported in the pre-print article were not included in the registry (length of hospital stay, length of ICU admission, death and intubation). The target sample size specified in the registry was not achieved. It is unclear if the study was registered prior to starting recruitment. There is no change from the trial registration in the intervention and control treatments. The mortality outcome reported was not found in the study registry. Adverse events were reported only in the intervention group. |
Trial *
Publication Kumari P, Cureus (2020) (published paper)
Dates: 2020-03-01 to 2020-07-30
Funding: No specific funding (no funding
"All authors have declared that no financial support was received from any organization for the submitted work.")
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / Pakistan Follow-up duration (days): * | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Vitamin C 50 mg/kg/day IV. |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Vitamin C=75 Standard care=75 | |
| Characteristics of participants N= 150 Mean age : NR 0 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=150 Critical: n=0 | |
| Primary outcome | |
| In the register NR | |
| In the report NR | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
* |
| General comment |
This study is pending contact with authors due to lack of information on outcome timepoints, and overall follow-up.
The published article was used in data extraction and risk of bias assessment. Trial registry, protocol, and statistical analysis plan were not available at the time of data extraction. The study reported on need for mechanical ventilation (12/75 vs. 15/75) and on overall mortality (7/75 vs. 11/75), however, these data have not been added to analyses because follow-up time-points were not reported. |
Trial NCT04264533
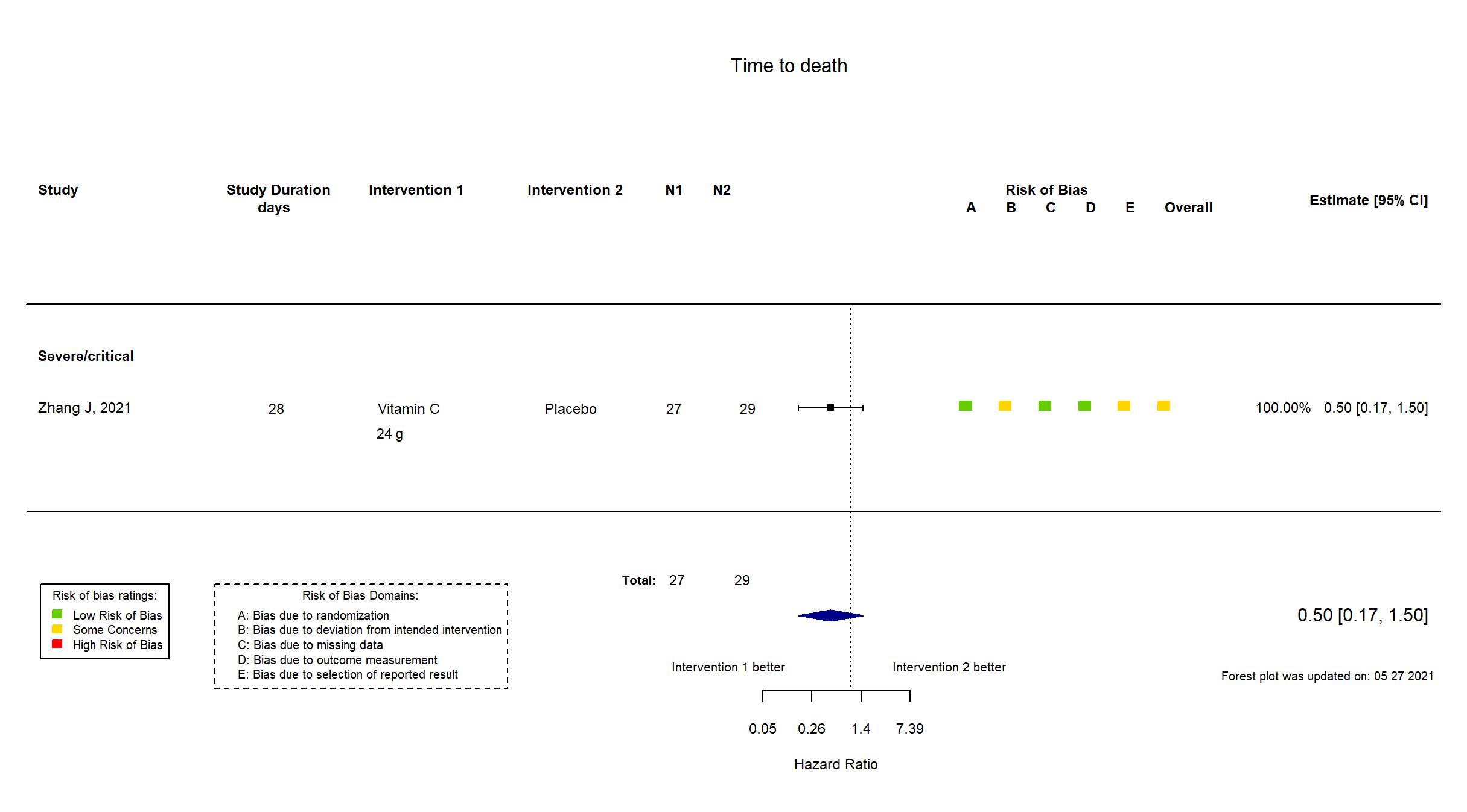
Publication Zhang J, Ann. Intensive Care (2021) (published paper)
Dates: 14/02/2020 to 29/03/2020
Funding: Public/non profit (Science and Technology Department of Hubei Province; Fundamental Research Funds for the Central Universities)
Conflict of interest: No
| Methods | |
| RCT Blinding: Participants, outcome assessor and health care pro | |
| Location :
Multicenter / China Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Vitamin C 12 mg diluted in 50 mL of bacteriostatic water IV every 12h at a rate of 12 mL/hour for 7 days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Vitamin C=27 Placebo=29 | |
| Characteristics of participants N= 56 Mean age : NR 37 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=* Critical: n=* | |
| Primary outcome | |
| In the register Ventilation-free days [ Time Frame: on the day 28 after enrollment ] | |
| In the report IMV- free days in 28 days | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the study report, the study protocol, the pre-print article and the study registry were used in data extraction and risk of bias assessment. The study was terminated early.
Quote: "With the control of the epidemic, this trial was stopped early, and the number of qualifying COVID-19 patients did not satisfy the anticipated sample size(140)" There is no change from the trial registration in the intervention and control treatments. The primary outcome in the registry matches that in the report. Some secondary outcomes specified in the registry (Demand for first aid measurements and APACHE II scores) were not specified in the pre-print report. The inverse is also true as some secondary outcomes in the report were not present in the registry (organ functions and inflammatory parameters, patient condition improvement rate, patient condition deterioration rate). This preprint was updated on 23/09/2020 and again on 20/01/2021 using the published report. |