Umbilical cord mesenchymal stem cell infusion vs Standard care/Placebo (RCT)
Hospitalized patients
FOREST PLOTS -2022-04-03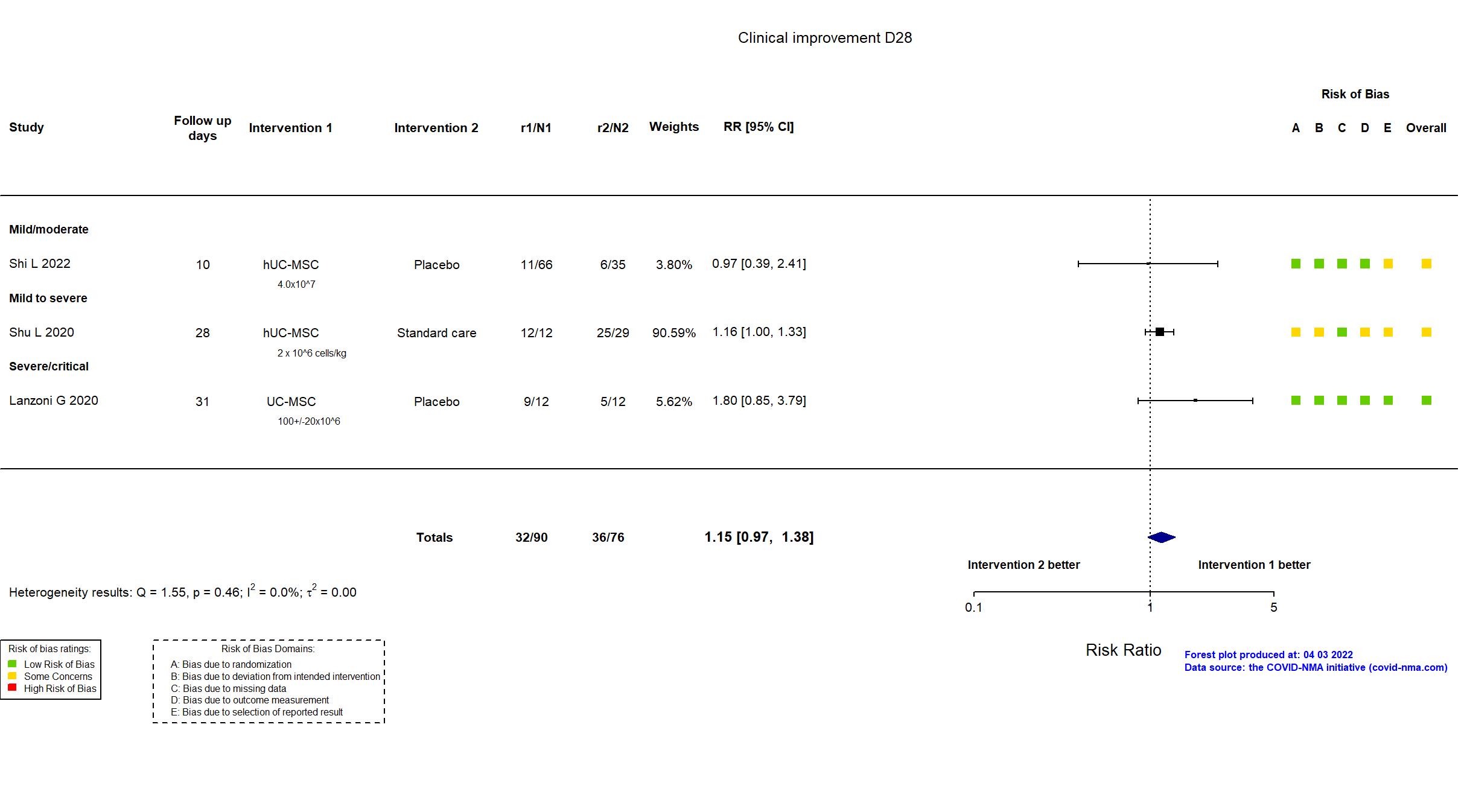

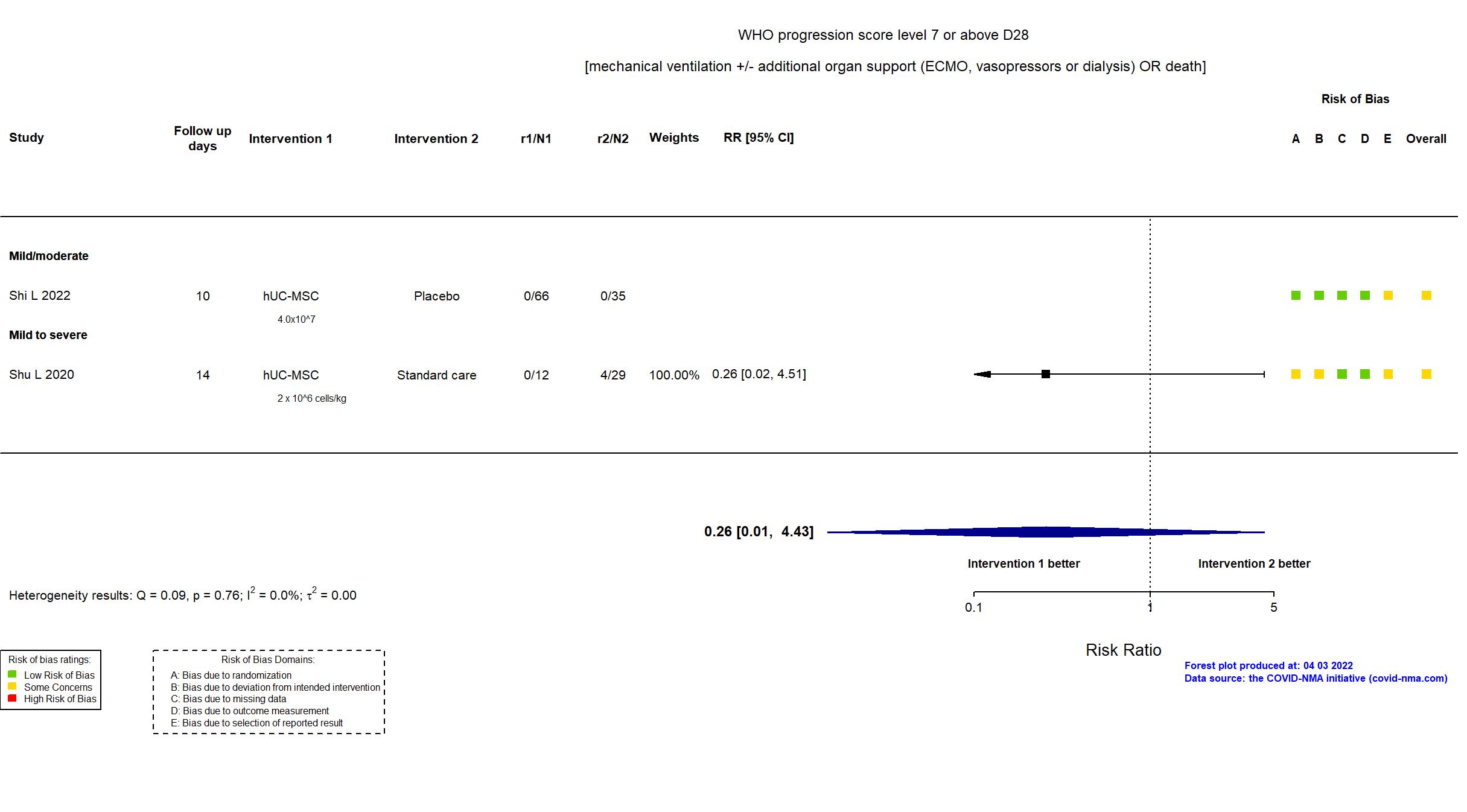
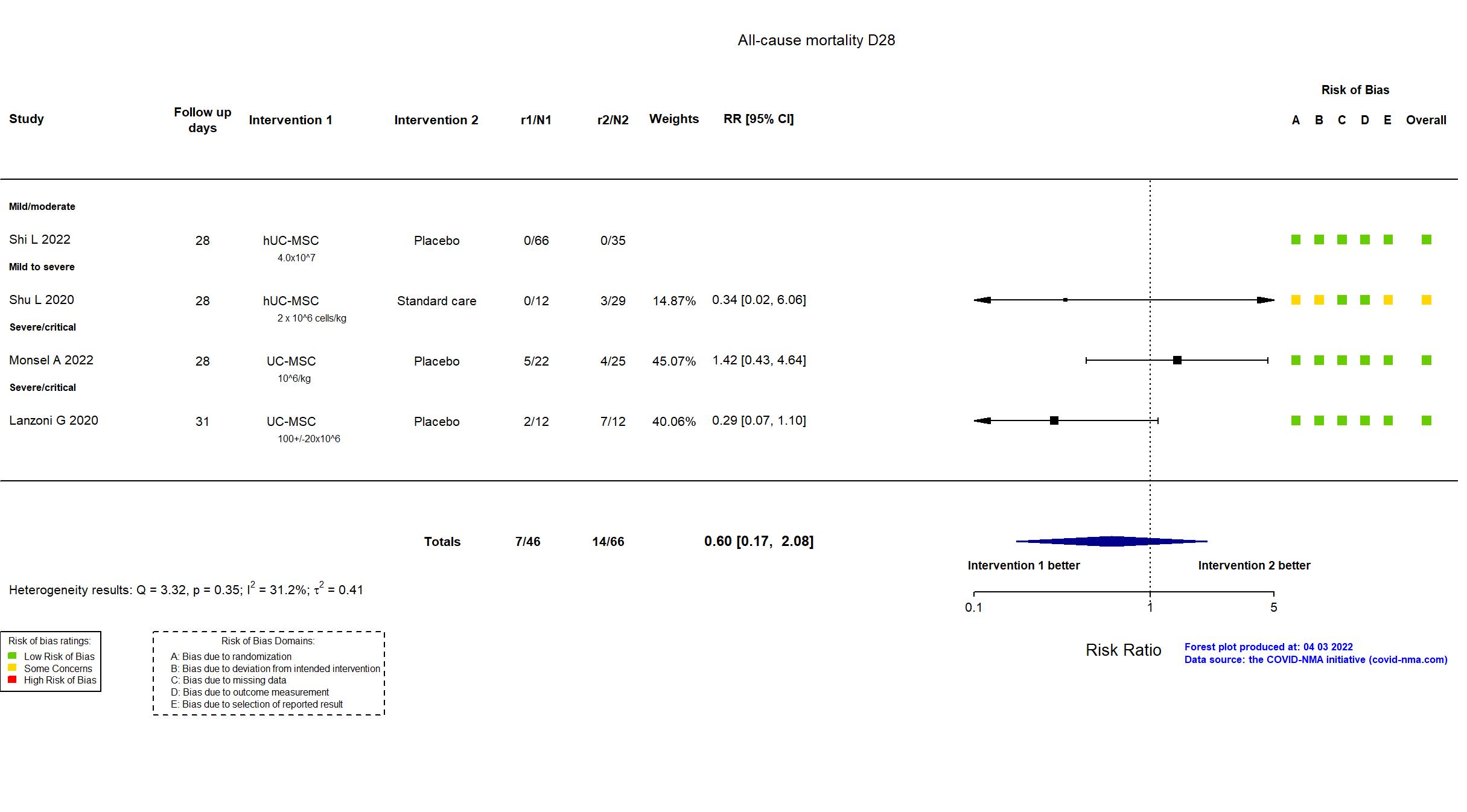
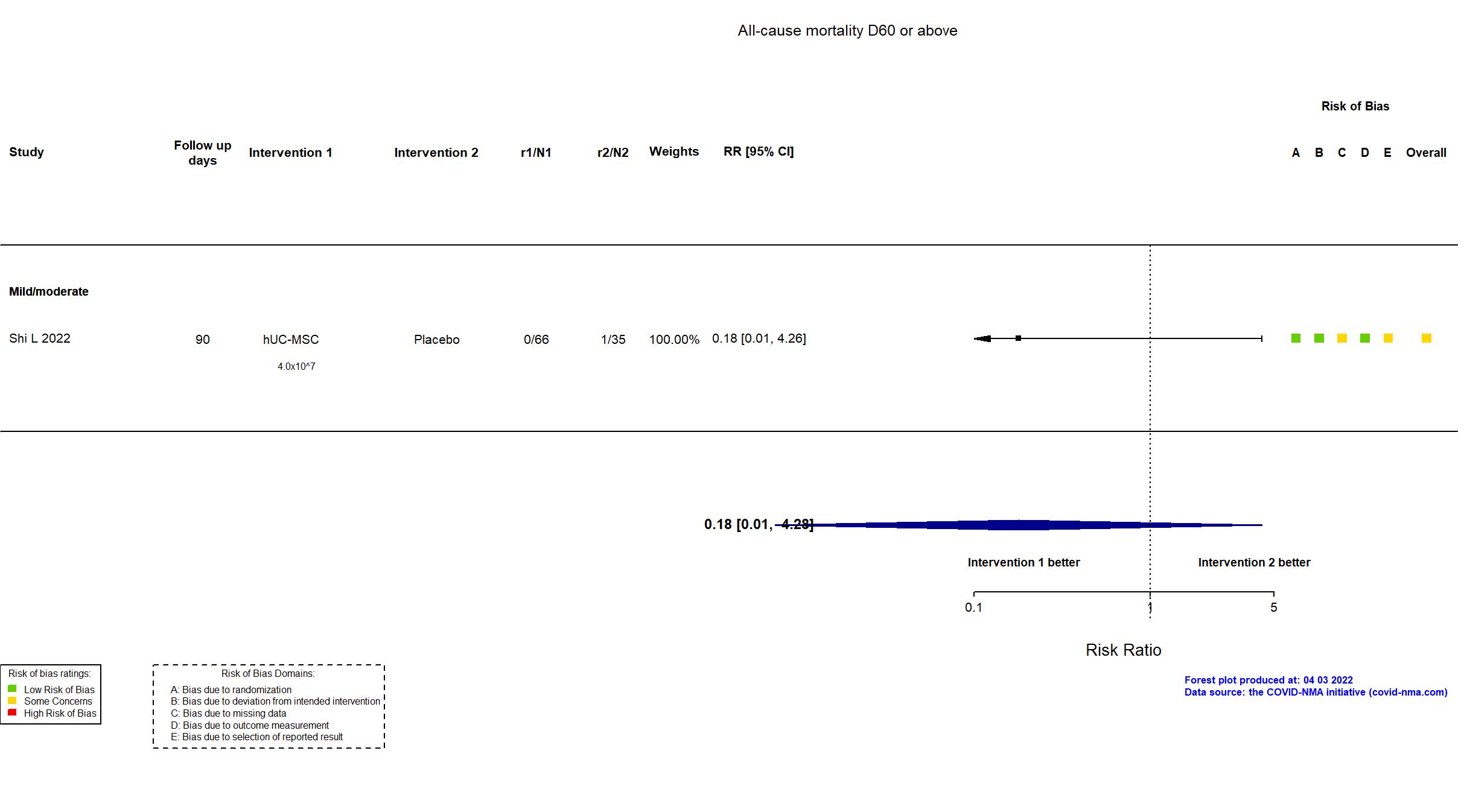
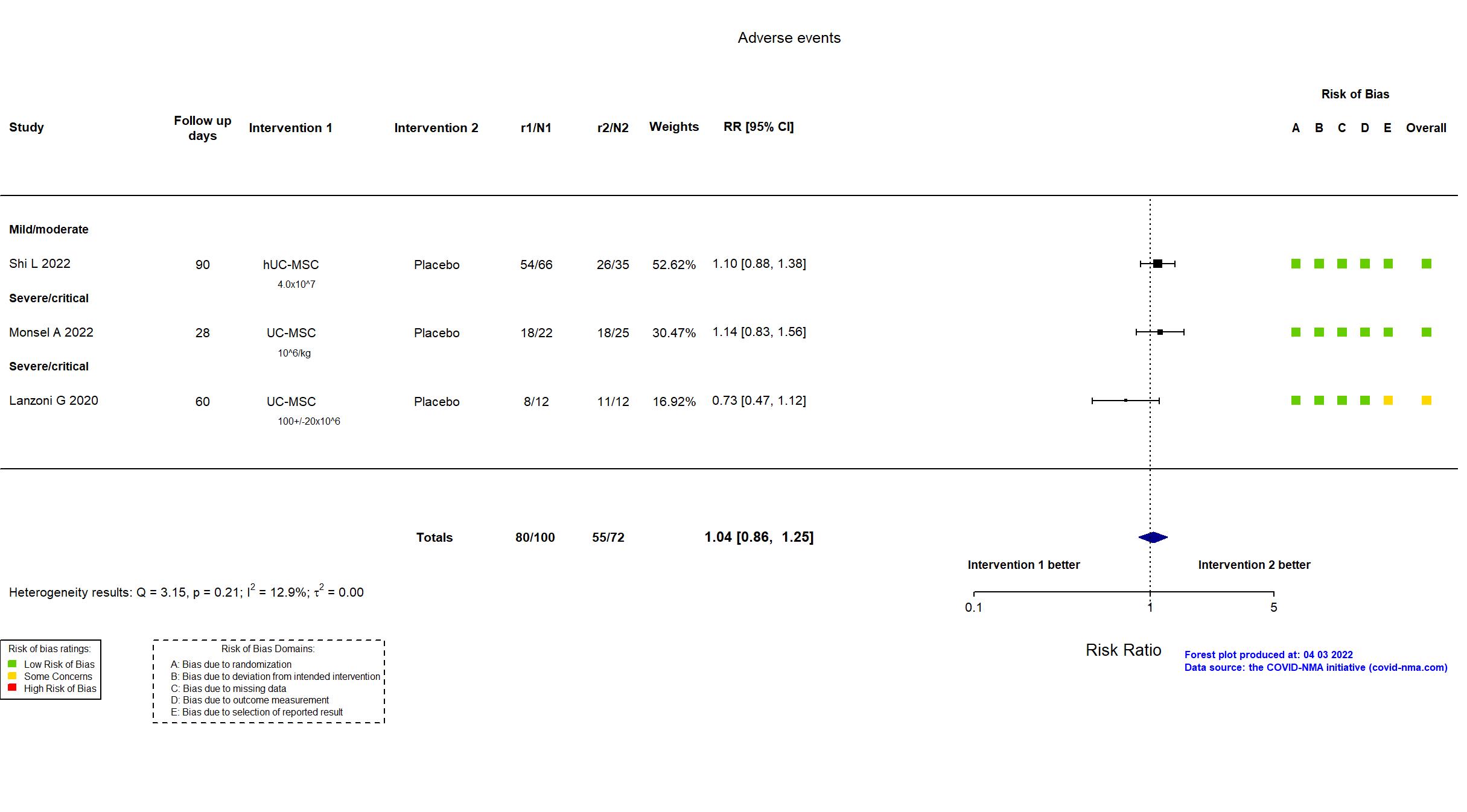
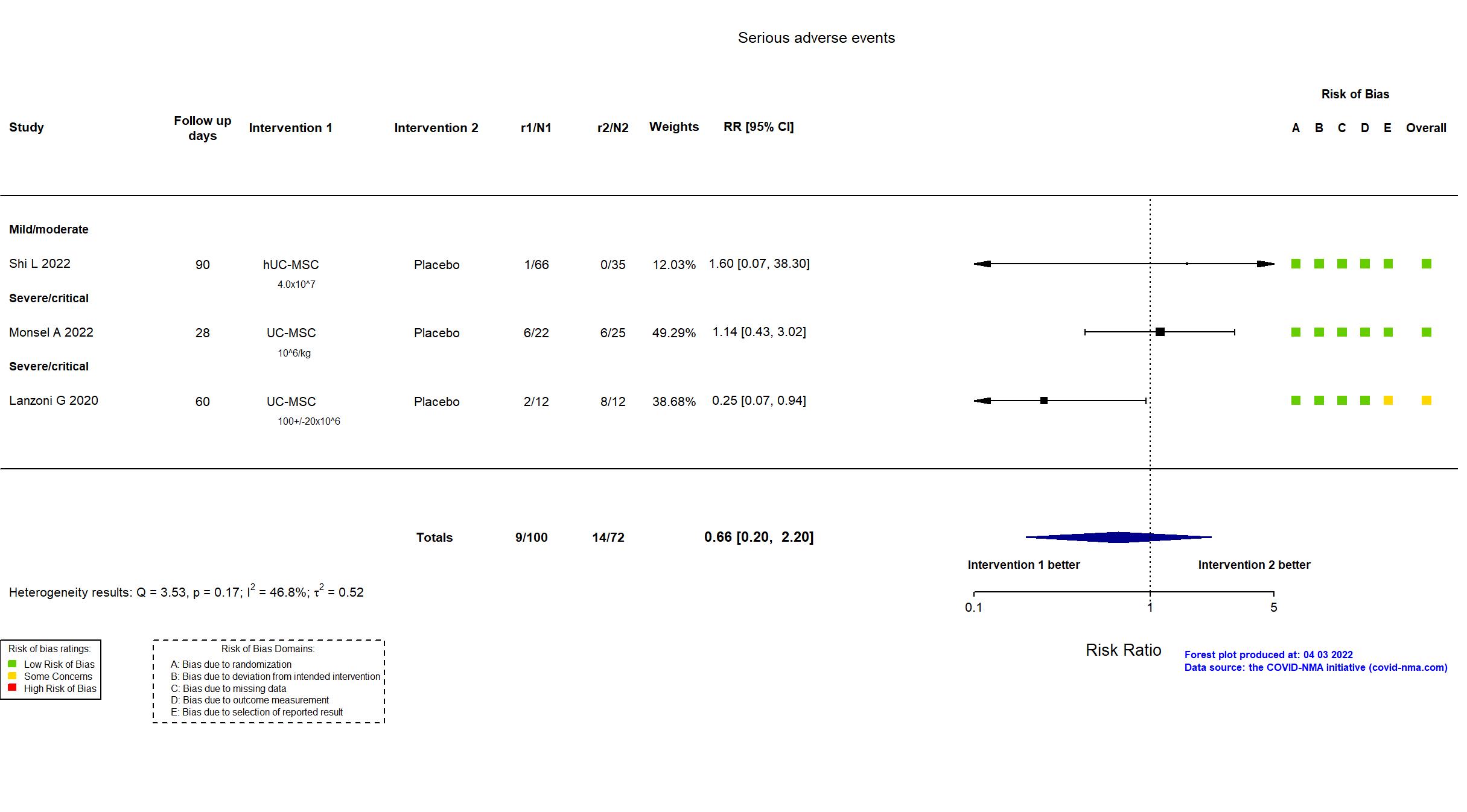
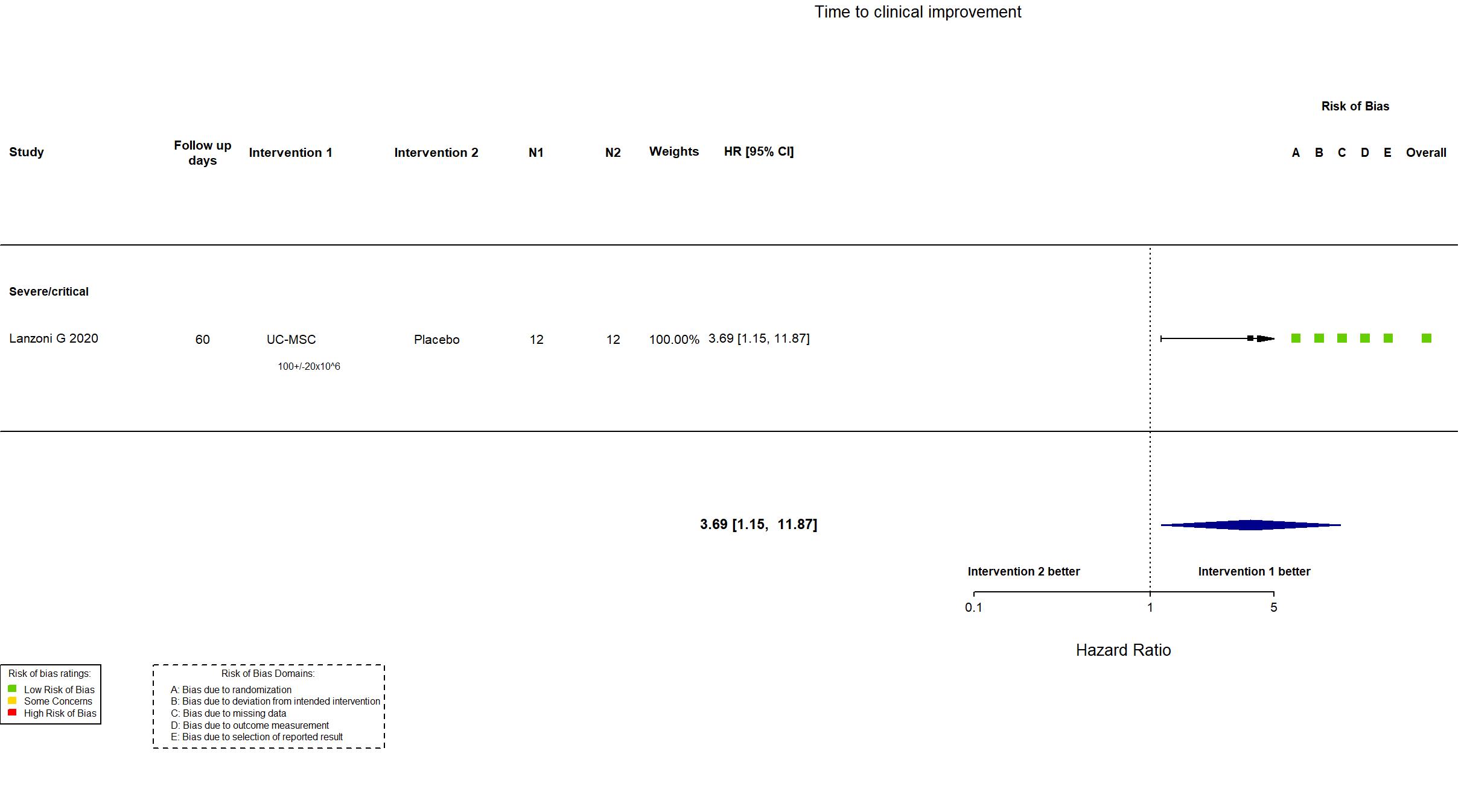
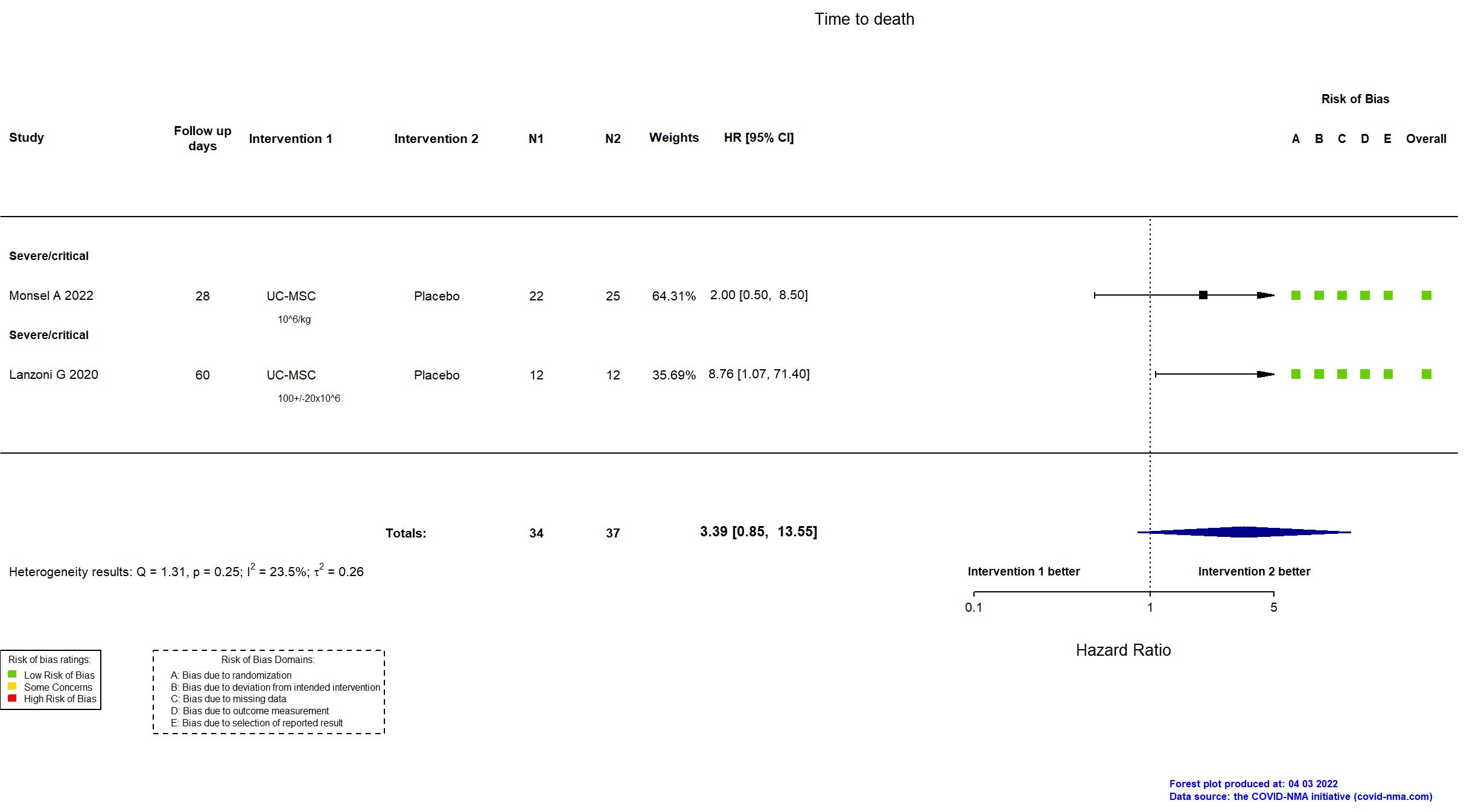









Trial NCT04457609
Publication Dilogo IH, Stem Cells Transl Me (2021) (published paper)
Dates: 2020-05-01 to 2020-10-10
Funding: Public/non profit (Ministry of Research and Technology/National Research and Innovation Agency
Republic of Indonesia.)
Conflict of interest: No
| Methods | |
| RCT Blinding: triple blinding | |
| Location :
Multicenter / Indonesia Follow-up duration (days): * | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
UC-MSC 1*10^6/kg IV, once-off |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Placebo=20 UC-MSC=20 | |
| Characteristics of participants N= 40 Mean age : NR 30 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=0 Critical: n=40 | |
| Primary outcome | |
| In the register Clinical improvement:Presence of dyspnea, presence of sputum, fever, ventilation status, blood pressure, heart rate, respiratory rate, oxygen saturation [Time Frame: 15 days] | |
| In the report mortality rate and length of ventilator usage | |
| Documents avalaible |
Protocol Yes. In English Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
* |
| General comment |
This study is pending author reply. Time points outcomes were not clearly reported.
In addition to the available version of the publication, the study registry and protocol were used in data extraction and risk of bias assessment. The study achieved the target sample size specified in the trial registry. There is no change from the trial registration in the intervention and control treatments. The registry primary outcome does not reflect the reported primary outcome. Some outcomes from the registry are not reported in the paper (e.g. clincal improvement).Some outcomes (e.g. mortality) are reported in the paper, but were not pre-specified in the trial registry. In the registry, the primary outcome is clinical improvement in the presence of dyspnea, presence of sputum, fever, ventilation status, blood pressure, heart rate, respiratory rate, and oxygen saturation. The follow-up time of study participants is unclear. |
Trial NCT04355728
Publication Lanzoni G, Stem Cells Transl Me (2021) (published paper)
Dates: 2020-04-25 to 2020-07-21
Funding: Mixed (Ugo Colombo;
Simkins Family Foundation; Fondazione Silvio
Tronchetti Provera; Barilla Group & Family; Diabetes Research Institute Foundation;
The Cure Alliance; NABTU; NCATS)
Conflict of interest: No
| Methods | |
| RCT Blinding: | |
| Location :
Single center / USA Follow-up duration (days): 60 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
UC-MSC 100 +/- 20x10^6 in a 50 mL vehicle solution containing human serum albumin and heparin, infused at days 0 and 3 |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : UC-MSC=12 Placebo=12 | |
| Characteristics of participants N= 24 Mean age : NR 13 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=13 Critical: n=11 | |
| Primary outcome | |
| In the register Incidence of pre-specified infusion associated adverse events [ Time Frame: Day 5 ] Incidence of Severe Adverse Events [ Time Frame: 90 days ] | |
| In the report Safety, as defined by the occurrence of pre-specified infusion associated AEs, occurring within 6 hours from each infusion; Cardiac arrest or death within 24 h post infusion; Incidence of AEs | |
| Documents avalaible |
Protocol Yes. In English Statistical plan NR Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published article, the pre-print article, the trial registry and supplementary appendix as well as responses from contact with authors were used in data extraction and assessment of risk of bias. The study protocol was referenced in the paper but no date was provided to determine its prospective or retrospective nature and aid in the risk of bias assessment for the domain 'Selection of Reported Result'.
There were no substantive differences between the pre-print article and the trial registry in population, procedures and interventions. There were some differences between the pre-print article and trial registry in secondary laboratory outcomes. On February 12th, 2021, we received additional information from authors on this study. This study was updated with data from contact with authors on March 30th, 2021. |
Trial NCT04333368, EudraCT 2020-001287-28
Publication Monsel A, Crit Care (2022) (published paper)
Dates: 2020-04-06 to 2020-10-29
Funding: Public/non profit (French Ministry of Health and the French National Research Agency)
Conflict of interest: No
| Methods | |
| RCT Blinding: triple blinding | |
| Location :
Multicenter / France Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
UC-MSC Three intravenous infusions of 10^6 UC-MSCs/kg (maximum dose set at 80 × 10^6 cells per infusion) or placebo on D1, D3 ± 1 and D5 ± 1. |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : UC-MSC=22 Placebo=25 | |
| Characteristics of participants N= 47 Mean age : NR 37 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=14 Critical: n=31 | |
| Primary outcome | |
| In the register Respiratory efficacy evaluated by the increase in PaO2/FiO2 ratio from baseline to day 7 in the experimental group compared with the placebo group | |
| In the report Respiratory improvement assessed as the partial pressure of oxygen to fractional inspired oxygen (PaO2/FiO2)-ratio change between baseline (D0) and D7 post-randomization. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Low |
| General comment | “In addition to the published article, the supplementary materials including protocol and study registry were used in data extraction and risk of bias assessment. There is no change from the trial registration in the intervention and control treatments. The registry primary outcome reflects the reported primary outcome. Some outcomes from the registry are not reported in the paper (but these are not relevant to our review). Adverse events reported separately for before and after Day 14, so only those up to Day 14 were extracted due to potential patient overlap. The study (n=47) did not achieve the target sample size (n=60) specified in the trial registry." |
Trial NCT04288102
Publication Shi L, eBioMedicine (2022) (published paper)
Dates: 2020-03-05 to 2021-03-31
Funding: Public/non profit (The National Key R&D Program of China; The Innovation Groups of the National Natural Science Foundation of China; The National Science and Technology Major Project)
Conflict of interest: No
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / China Follow-up duration (days): 90 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
hUC-MSC 4.0x10^7 MSCs in a volume of 100 mL, infused on days 0, 3 and 6. |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : hUC-MSC=66 Placebo=35 | |
| Characteristics of participants N= 101 Mean age : NR 56 males Severity : Mild: n=24 / Moderate: n=75 / Severe: n=1 Critical: n=0 | |
| Primary outcome | |
| In the register Change in lesion proportion (%) of full lung volume from baseline to day 28 | |
| In the report change in the total lesion proportion (%) of the whole lung volume from baseline to day 28, as measured by chest CT | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published/pre-print article, the trial registry, study protocol, supplementary materials and statistical analysis plan were used in data extraction and assessment of risk of bias. Originally planned as a Phase 1 study, the study progressed to Phase 2 with an expanded sample size due to satisfactory assessment of safety. The study exceeded its original and amended sample sizes. Changes were made to primary outcome measure in the trial registry both during and after study conduct, but this is reported along with the reasons in the pre-print article. Some outcomes from the registry/protocol are not reported in the paper (e.g., time to clinical improvement).
This study was updated on March 19th, 2021 using data from the published manuscript (Signal Transduction). This study was updated on March 22nd, 2022 using 1-year follow-up data from the published manuscript (eBioMedicine) |
Trial ChiCTR2000031494
Publication Shu L, Stem Cell Res Ther (2020) (published paper)
Dates: 12/02/2020 to 25/03/2020
Funding: Public/non profit (National Natural Science Foundation of China; Key Research and Development Project of Jiangsu Province)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / China Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
hUC-MSC 2 x 10^6 cells/kg IV in 100 mL of normal saline once off. |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : hUC-MSC=12 Standard care=29 | |
| Characteristics of participants N= 41 Mean age : NR 24 males Severity : Mild: n=3 / Moderate: n=28 / Severe: n=10 Critical: n=0 | |
| Primary outcome | |
| In the register Chest Imaging; Lung Function; ADL | |
| In the report Incidence of progression from severe to critical illness and the time to a clinical improvement of two points on a seven-category ordinal scale | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | "In addition to the published article, the study registry was used in data extraction and risk of bias assessment. This is a report on a single centre pilot study. The target sample size of 18 specified in the registry was not achieved for the intervention arm. There is no change from the trial registration in the intervention and control treatments. The inclusion and exclusion criteria in the registry and report do not match. The primary outcome and all secondary outcomes from the report are not specified in the trial registry." |