Auxora vs Standard care/Placebo (RCT)
Hospitalized patients
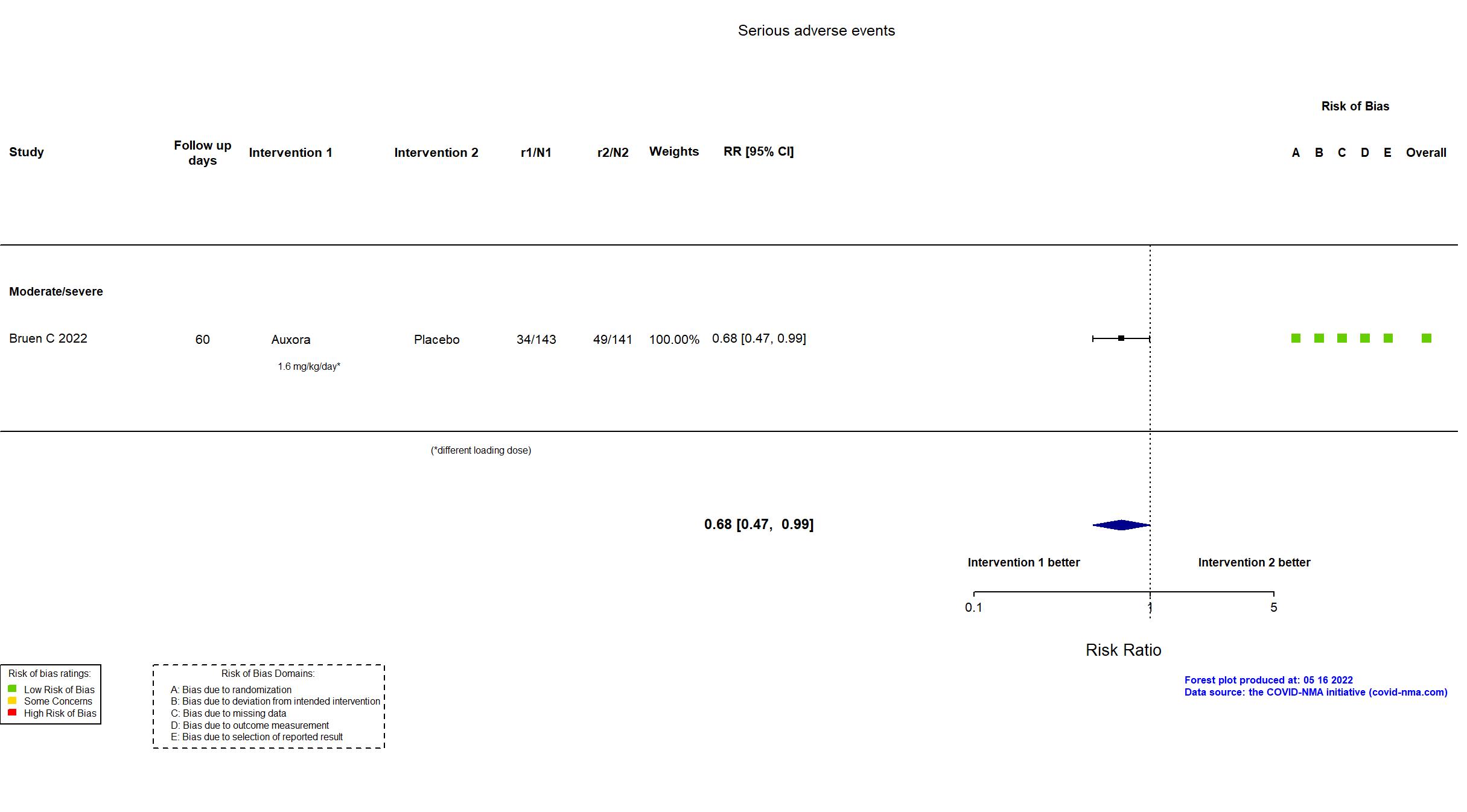
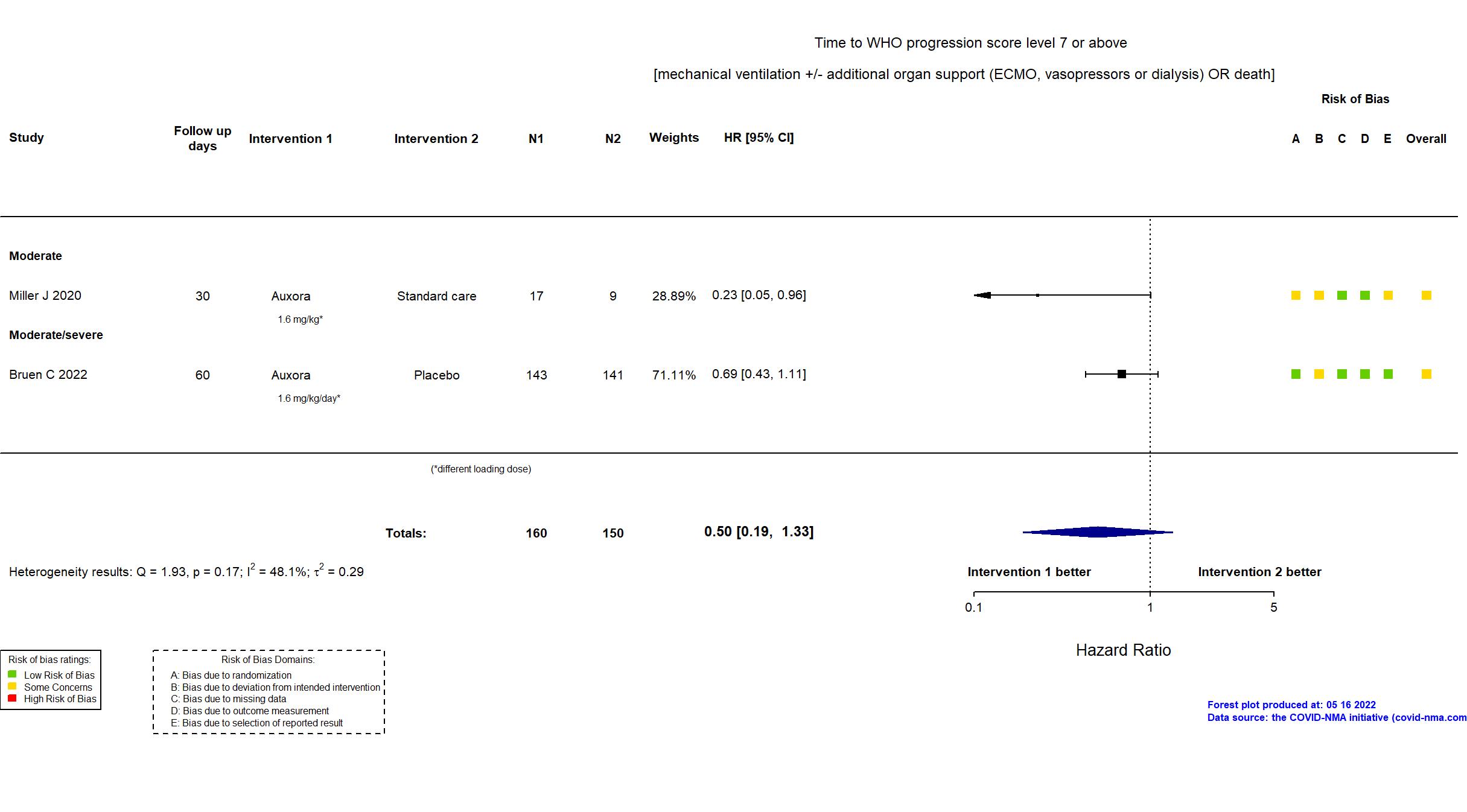
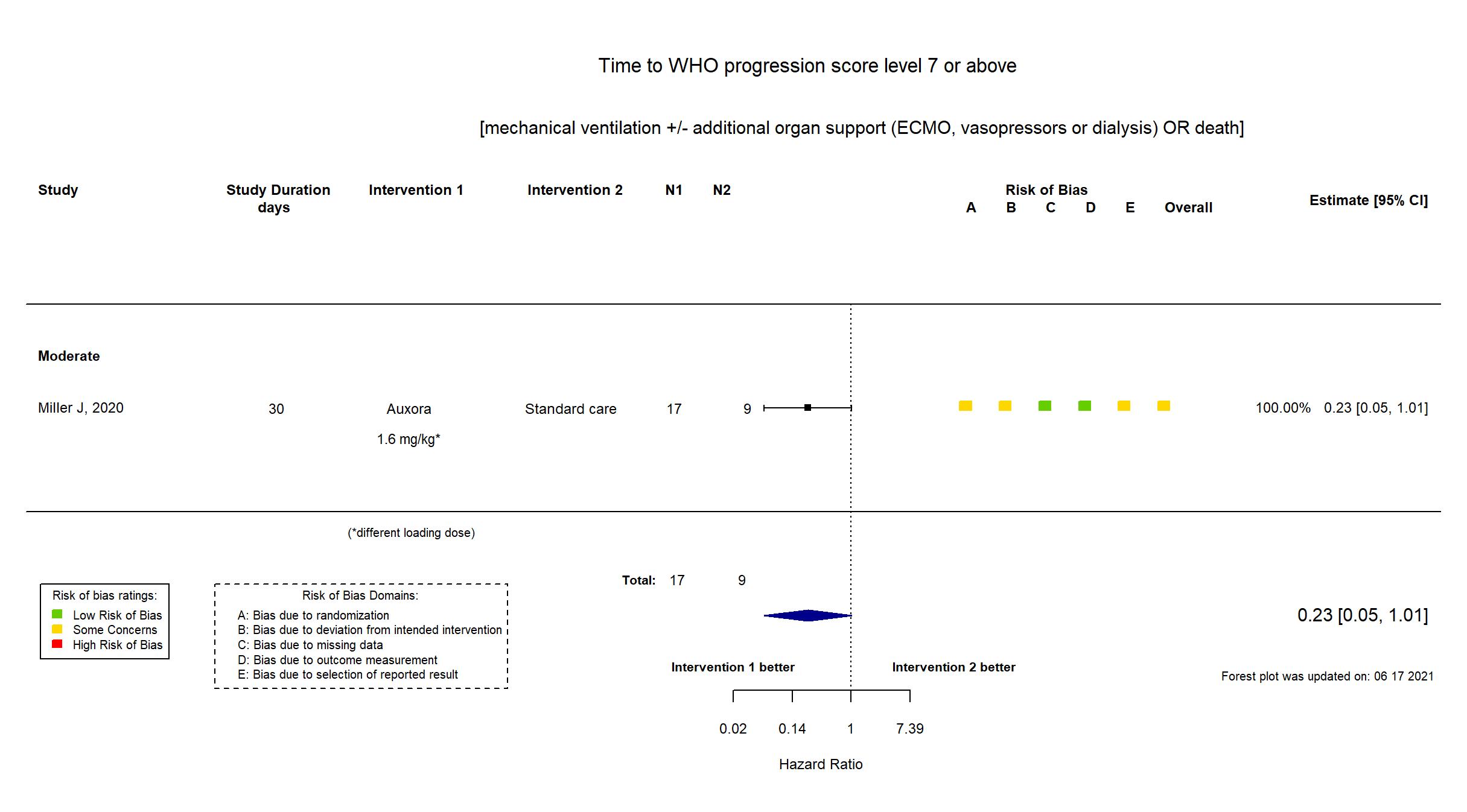
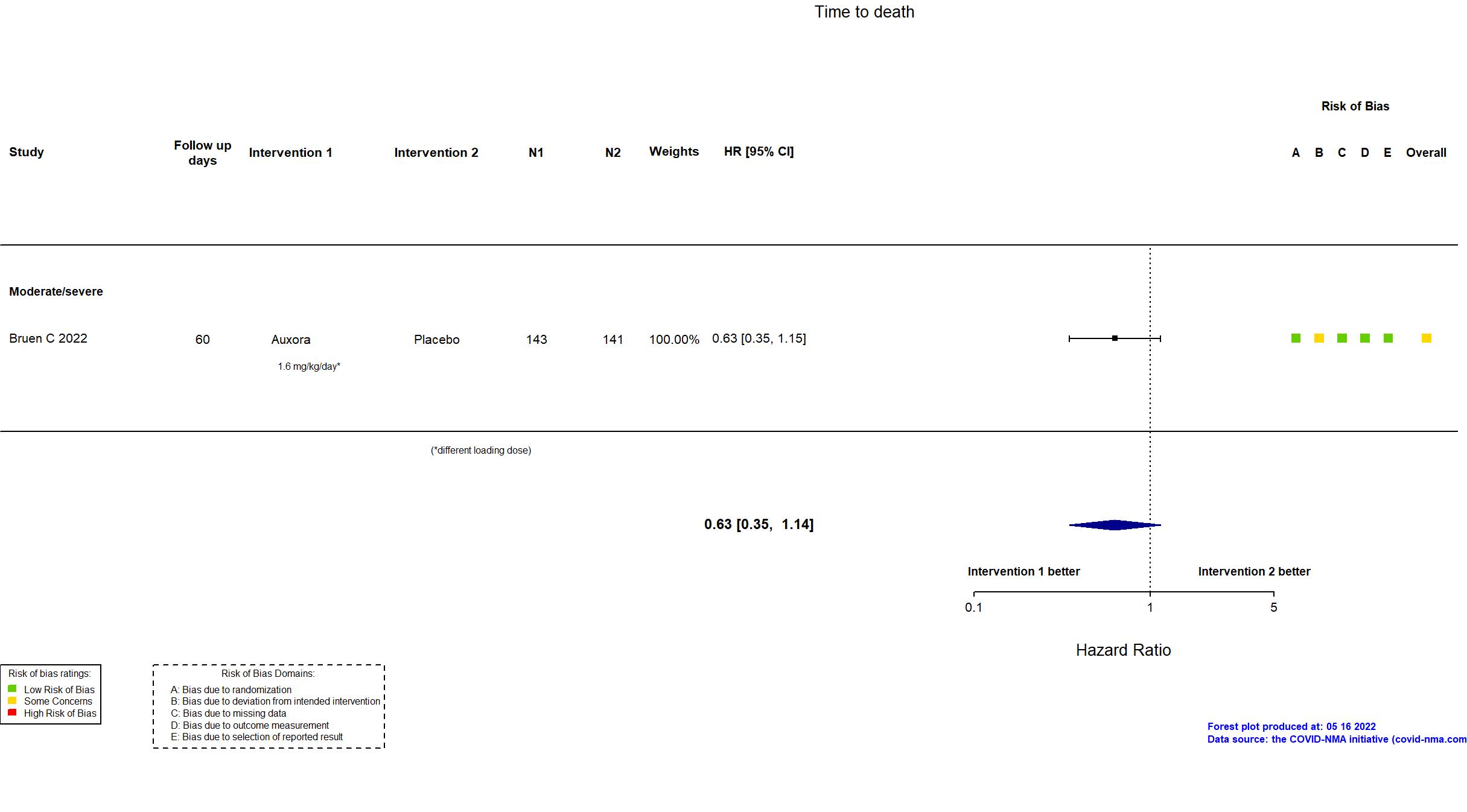
FOREST PLOTS -2022-05-16











Trial NCT04345614
Publication CARDEA - Bruen C, Crit Care (2022) (published paper)
Dates: 2020-09-08 to 2021-05-24
Funding: Private (CalciMedica, Inc. (La Jolla, CA, USA))
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / USA Follow-up duration (days): 60 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Auxora Initial dose: 2.0 mg/kg IV on day 1 - Maintenance dose: 1.6 mg/kg IV on days 2 and 3 |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Placebo=141 Auxora=143 | |
| Characteristics of participants N= 284 Mean age : NR 190 males Severity : Mild: n=0 / Moderate: n=98 / Severe: n=164 Critical: n=0 | |
| Primary outcome | |
| In the register Number of days from the Start of the First Infusion of Study Drug (SFISD) to recovery [ Time Frame: From start of first infusion of study drug to day 60 ] | |
| In the report Time to recovery through Day 60, defined as meeting the criteria for category 6 (Hospitalized, not requiring supplemental oxygen or ongoing medical care), category 7 (Discharged, requiring supplemental oxygen), or category 8 (Discharged, not requiring supplemental oxygen) using an 8-point ordinal scale. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published article, pre-print article, the protocol, the SAP, the supplementary material, the study registry and data from contact with authors were used in data extraction and risk of bias assessment. The study terminated early due to declining rates of US COVID-19 hospitalizations in the spring of 2021, and the adoption of tocilizumab as standard of care in patients at many study sites. Consequently. The trial (n = 284) did not achieve its target sample size (n = 400). There is no change from the trial registration in the intervention and control treatments. The registry primary outcome reflects the reported primary outcome. The study was first posted to SSRN but was later removed.
The study was updated on February 2nd, 2022 with data from the Research Square report. The study was updated on April 28th, 2022 with data acquired after contact with authors. The study was updated on May 11th, 2022 with data from the published report. |
Trial NCT04345614
Publication Miller J, Crit Care (2020) (published paper)
Dates: 2020-04-08 to 2020-05-13
Funding: Private (CalciMedica, Inc. )
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / USA Follow-up duration (days): 30 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Auxora Initial dose: 2.0 mg/kg IV infusion, maximum 250 mg followed by 1.6 mg/kg IV infusion, maximum 200 mg the next 2 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Standard care=9 Auxora=17 | |
| Characteristics of participants N= 26 Mean age : NR 12 males Severity : Mild: n=0 / Moderate: n=26 / Severe: n=0 Critical: n=0 | |
| Primary outcome | |
| In the register Number of days from the Start of the First Infusion of Study Drug (SFISD) to recovery [ Time Frame: From start of first infusion of study drug to day 30 ] | |
| In the report recovery rate defined as the first day the patient satisfied criterion 6, 7, or 8 of the 8-point ordinal scale | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to all available versions of the published article, the study registry and supplementary appendix were used in data extraction and risk of bias assessment. The study was terminated early by the US Food and Drug Administration. As a result, the target sample size specified in the registry was not achieved. Quote "The FDA provided guidance on May 12, 2020, to limit further enrolment in the open-label study and transition to a randomized, blinded, placebo-controlled study, and as such, both arms A and B ceased further enrolment". The study reported the results in two arms based on severity of the disease. Data on the arm with patients requiring high flow oxygenation (Arm B) was not used in this review as the sample size (n=4) was too small. In the trial registration, the control treatment was intended to be Placebo but was Standard care in the published report. There was no change from the trial registration in the intervention and control treatments in the primary outcome. Some secondary outcomes (number of days in the ICU and CM4620-IE serum concentration) were reported in the registry and not in the published article. |