Sargramostim vs Standard care (RCT)
Hospitalized patients
FOREST PLOTS -2022-09-04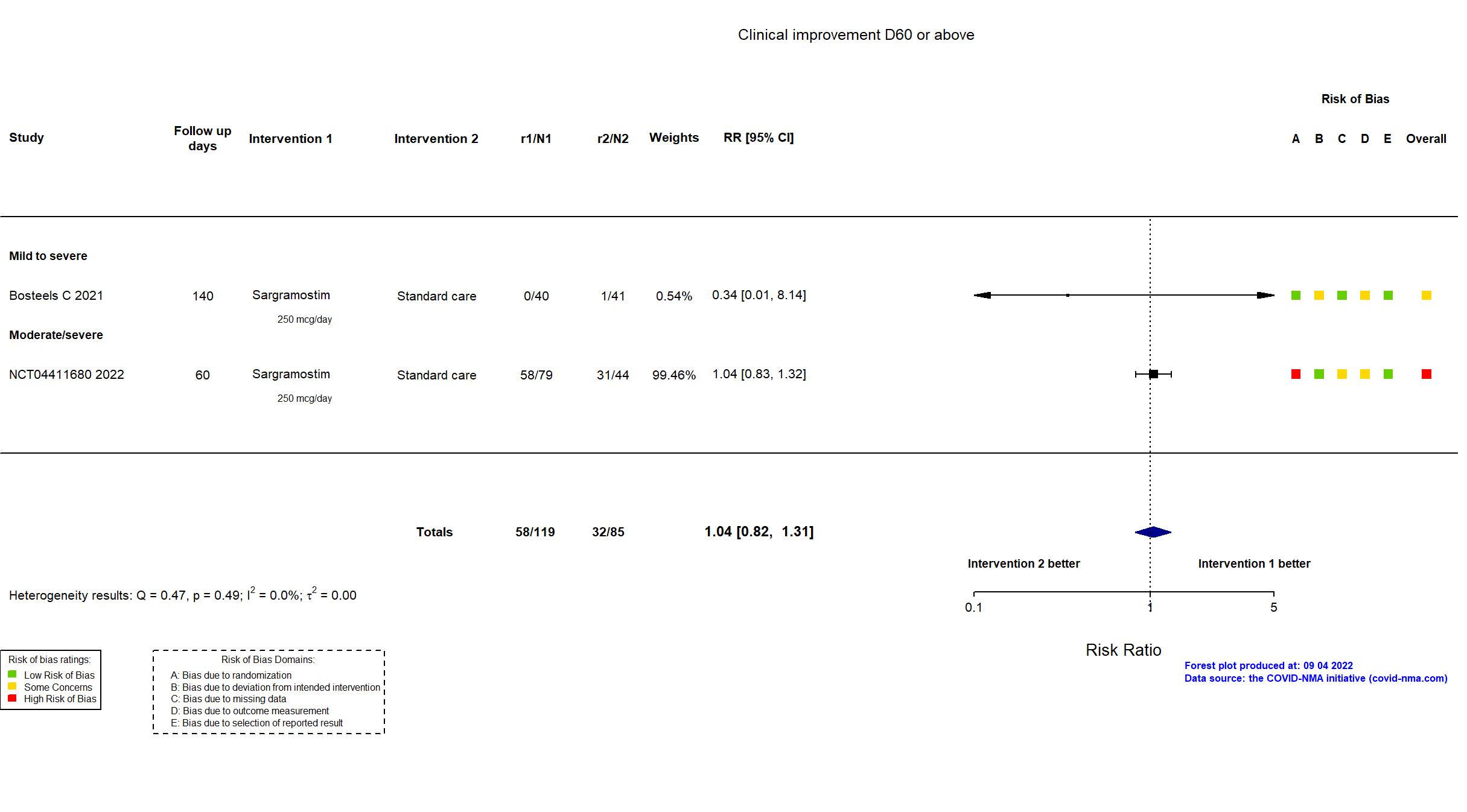

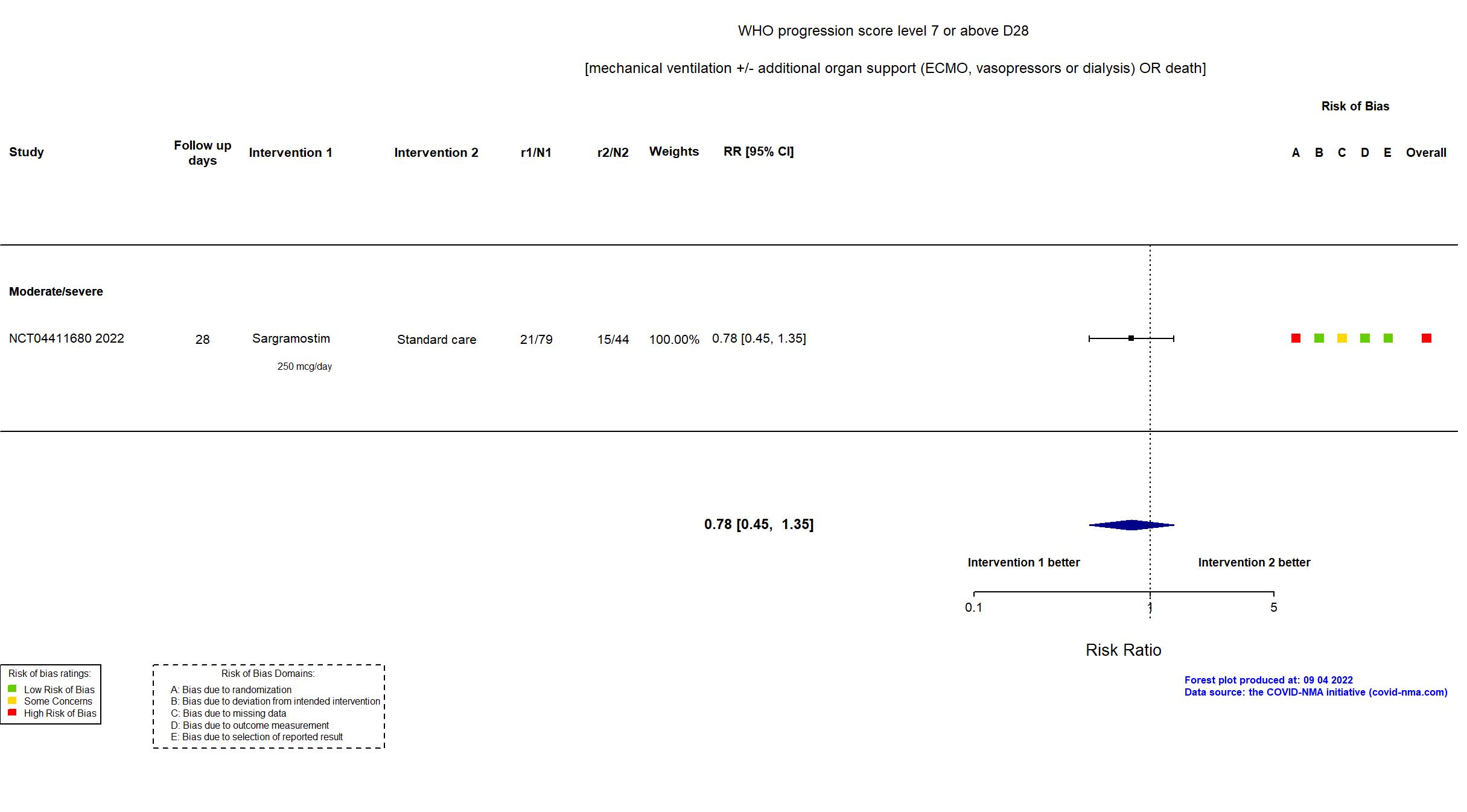
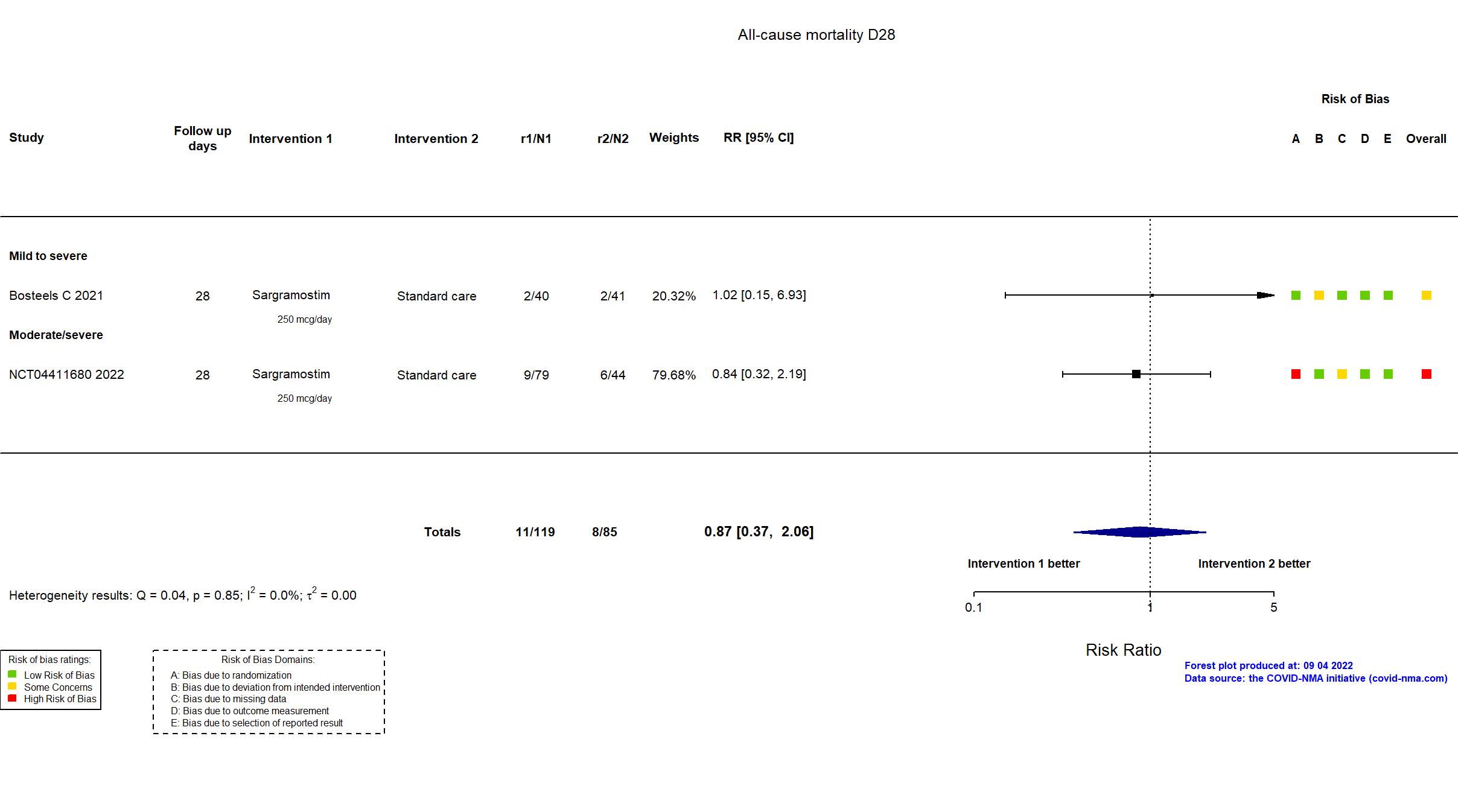
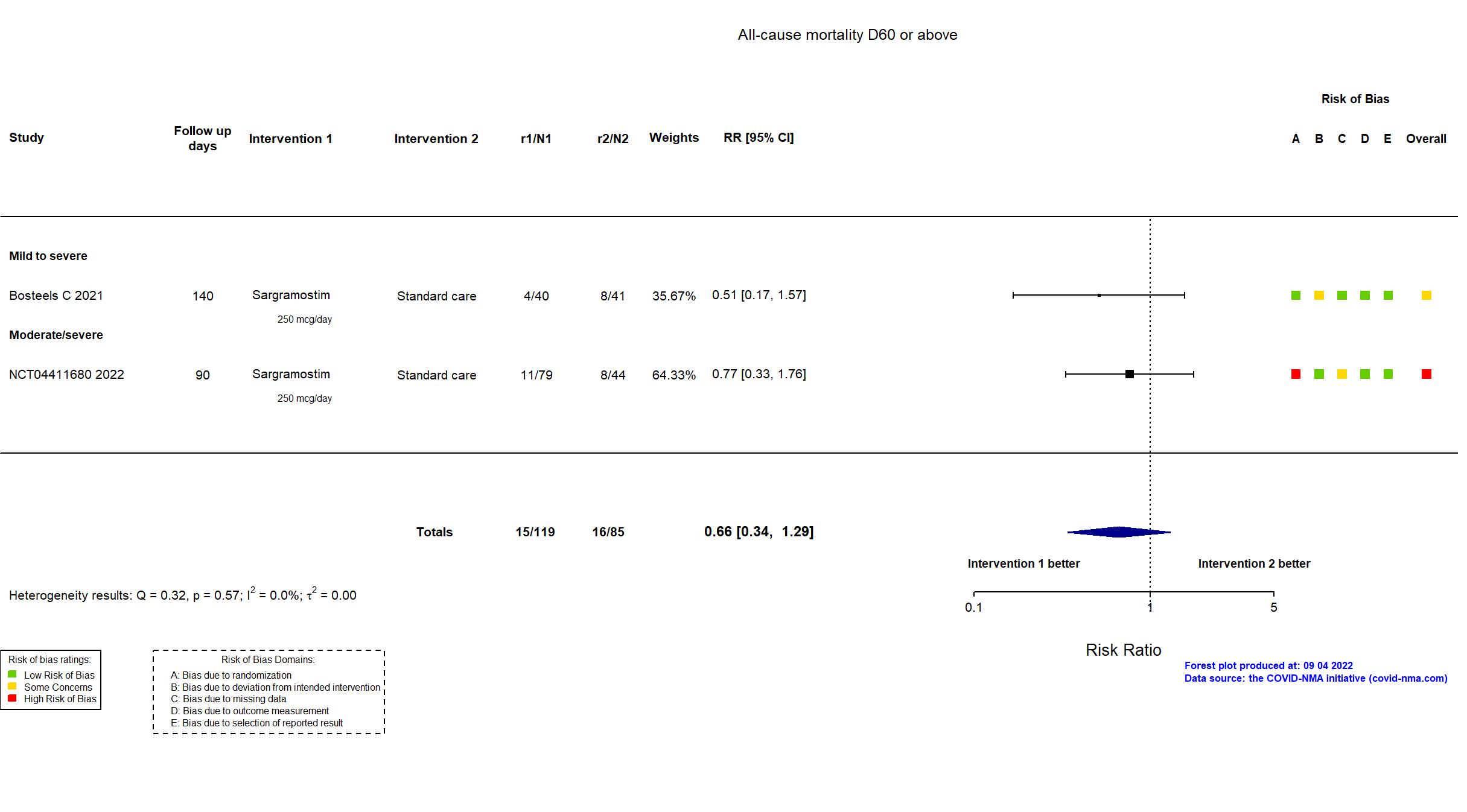
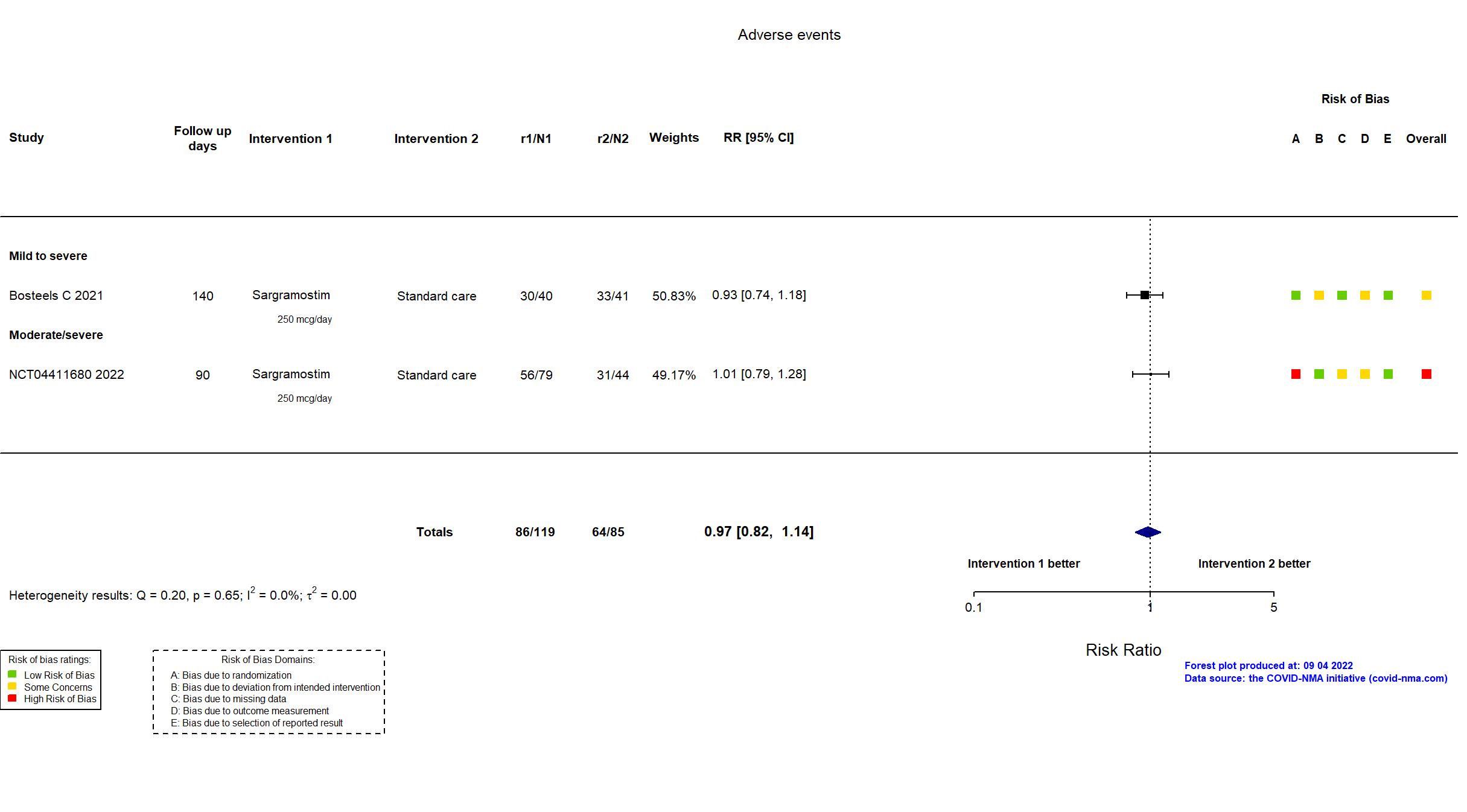
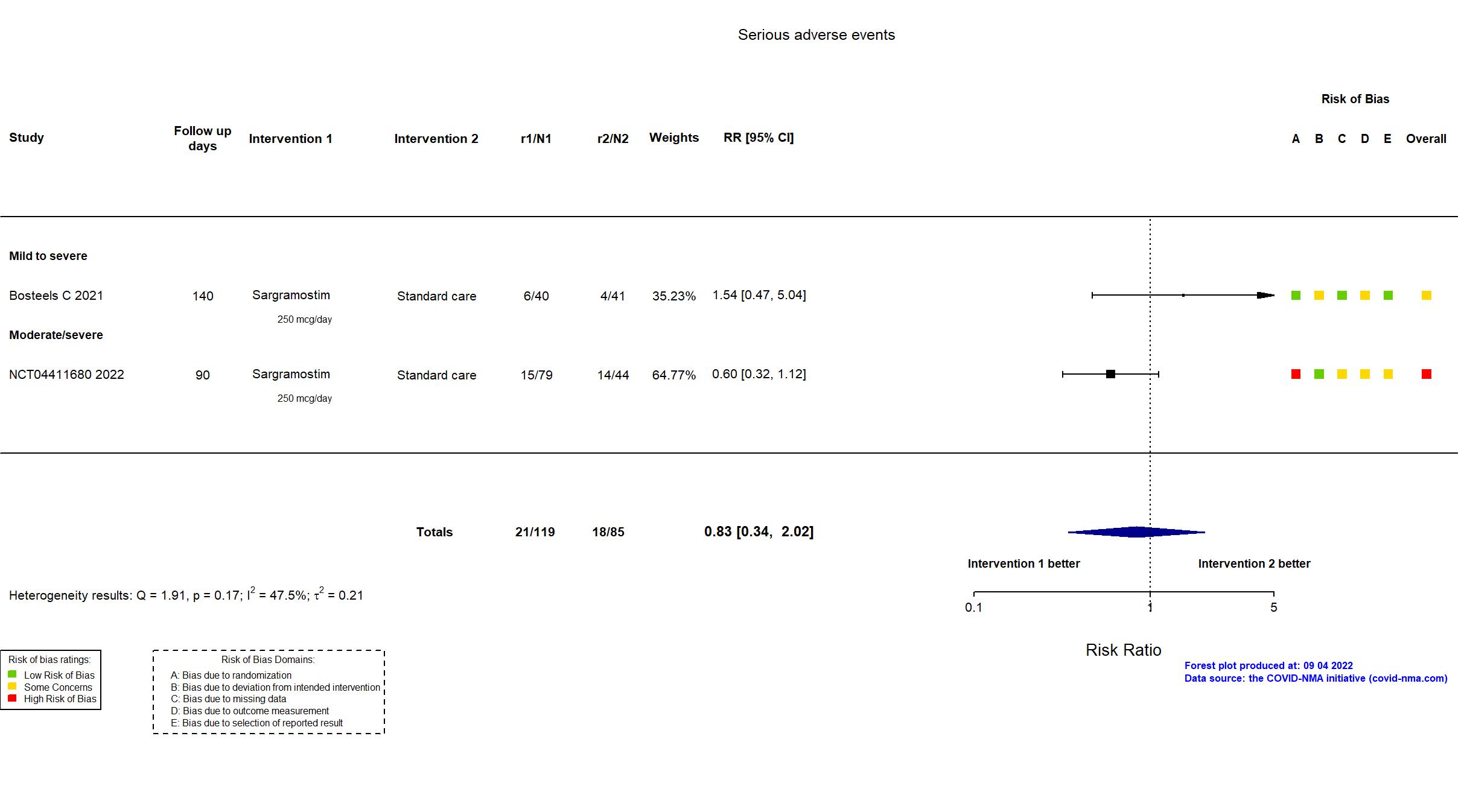
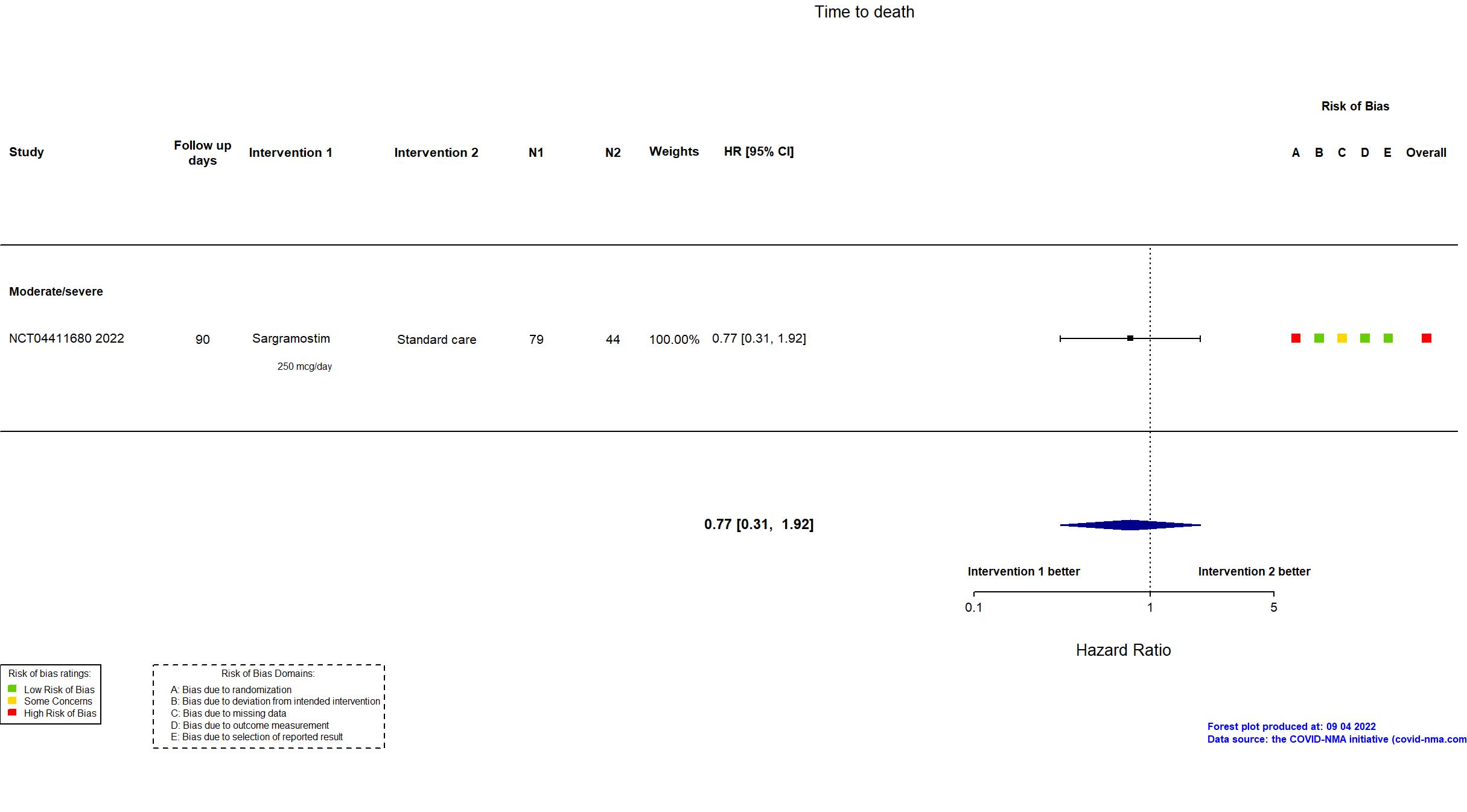








Trial NCT04326920
Publication Bosteels C, ResearchSquare (2021) (preprint)
Dates: 2020-03-25 to 2020-09-28
Funding: Mixed (Ghent University Hospital; Partner Therapeutics LLC (Lexington, MA) provided the study medication; The Ghent University Special Research Fund; VIB Grand Challenges program (Flanders Institute for Biotechnology); Chan Zuckerberg Initiative.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Belgium Follow-up duration (days): 140 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Sargramostim 125 mcg nebulized inhalation twice a day for 5 days (if intubated replaced by 125 μg/m2 body surface area intravenously once daily ) |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Standard care=41 Sargramostim=40 | |
| Characteristics of participants N= 81 Mean age : NR 51 males Severity : Mild: n=4 / Moderate: n=71 / Severe: n=6 Critical: n=0 | |
| Primary outcome | |
| In the register Improvement in oxygenation at a dose of 250 mcg daily during 5 days improves oxygenation in COVID-19 patients with acute hypoxic respiratory failure [ Time Frame: at end of 5 day treatment period ] by mean change in PaO2/FiO2 (PaO2=Partial pressure of oxygen; FiO2= Fraction of inspired oxygen) | |
| In the report Improvement in oxygenation after 5 days of sargramostim treatment and/or standard of care. Oxygenation was assessed by the PaO2/FiO2 ratio and P(Aa)O2 gradient. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the pre-print article, the trial registry, published and full protocols, statistical analysis plan and supplementary appendices were used in data extraction and assessment of risk of bias. The primary outcome in the article reflected that in the registry. The study (n = 81) achieved its target sample size (n = 80).
The study was updated on December 17th, 2021 with additional data from authors. |
Trial NCT04411680
Publication iLeukPulm - NCT04411680, Unpublished (2022) (results posted on registry)
Funding: Mixed (Partner Therapeutics, Inc, Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense's (JPEO-CBRND) Joint Project Manager for Chemical, Biological, Radiological, and Nuclear Medical (JPM CBRN Medical), under project agreement MCDC2006-012)
Conflict of interest: *
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / USA Follow-up duration (days): 90 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Sargramostim 125 mcg inhaled twice daily for 5 days or 125mcg/m2 intravenously every day for 5 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Sargramostim=79 Standard care=44 | |
| Characteristics of participants N= 123 Mean age : NR 61 males Severity : Mild: n=0 / Moderate: n=* / Severe: n=44 Critical: n=0 | |
| Primary outcome | |
| In the register 1. Change in Oxygenation Parameter of P(A-a)O2 Gradient by Day 6 [ Time Frame: 1-6 days ] The P(A-a)O2 gradient is a measure of how well the oxygen moves from the lungs into the bloodstream. Patients with a high gradient have less oxygen in the bloodstream. 2. Number of Patients Who Have Been Intubated by Day 14 [ Time Frame: 1-14 days ] | |
| In the report NR | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
High |
| General comment |
The trial registry, protocol and statistical analysis plane were used in data extraction and assessment of risk of bias. This is an unpublished study whose results have been reported in ClinicalTrials.gov. The trial was registered prospectively and no important changes were made to primary or secondary outcomes after recruitment start. The trial (n = 123) achieved its target sample size (n = 120).
Trial data were updated on September 1st, 2022 with data extracted after contact with authors. |