Dexamethasone Low Dose vs Dexamethasone High Dose (RCT)
Hospitalized patients
Studies included but not extracted/included in the analysis: Wu H, PloS one, 2022
FOREST PLOTS -2022-08-04
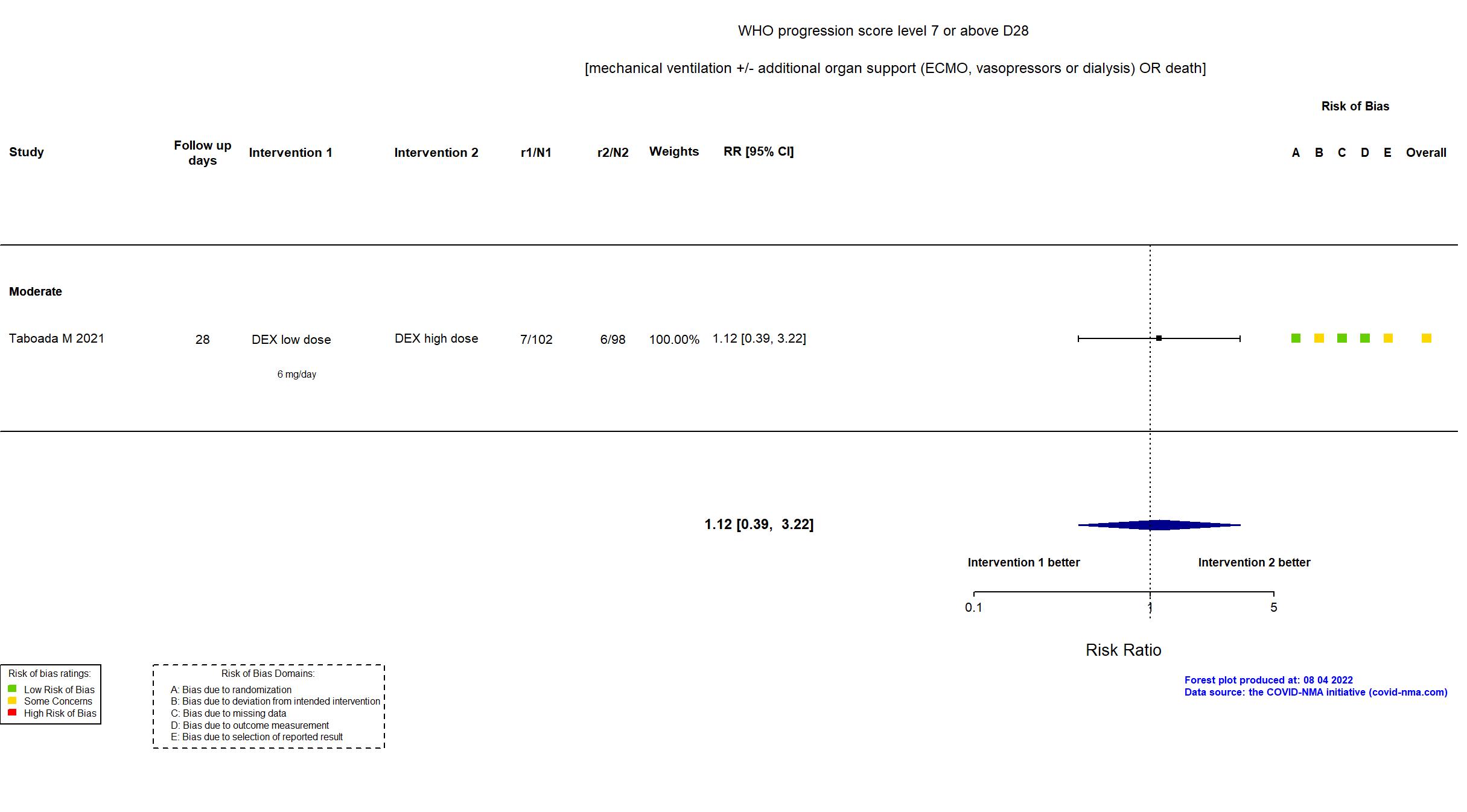
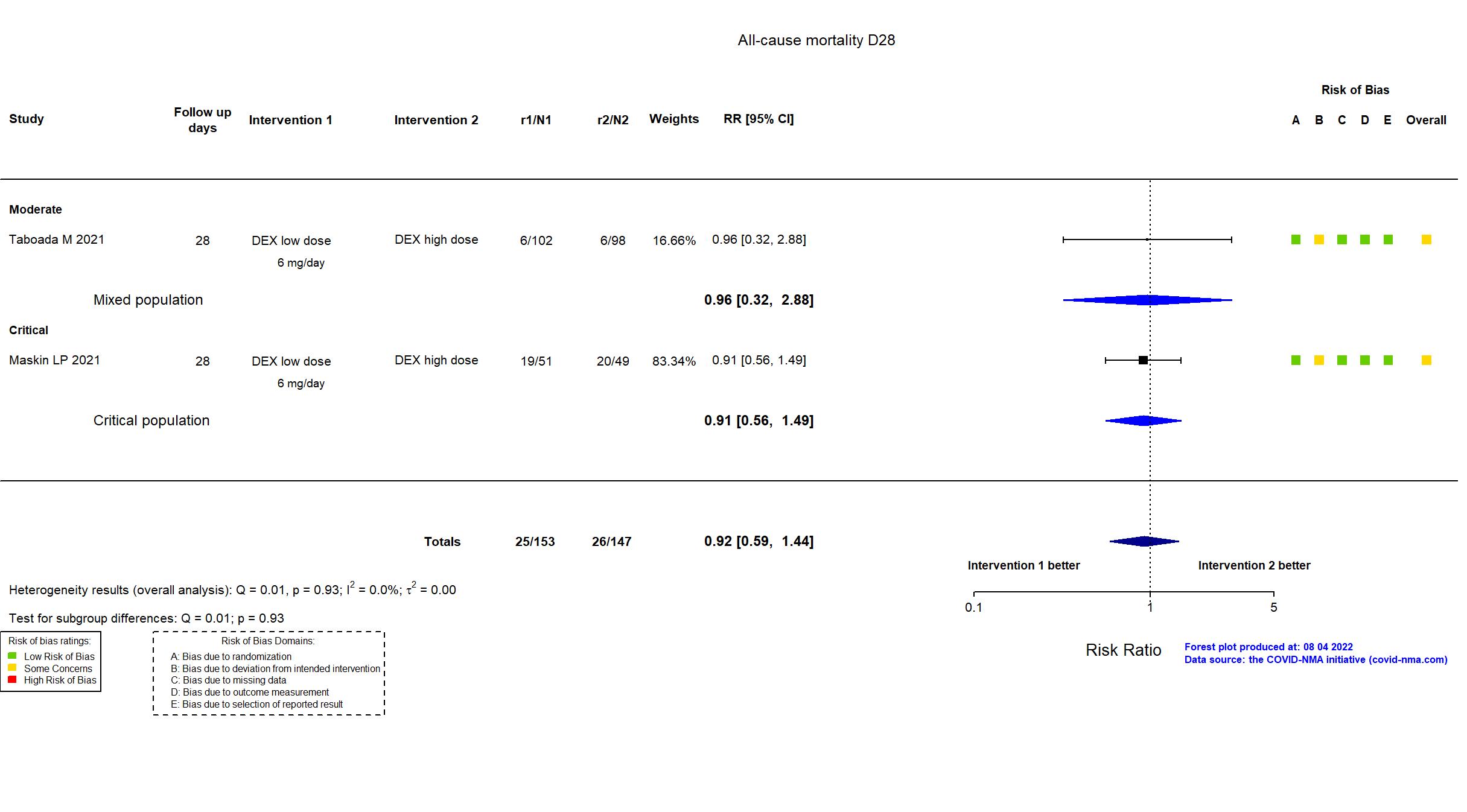
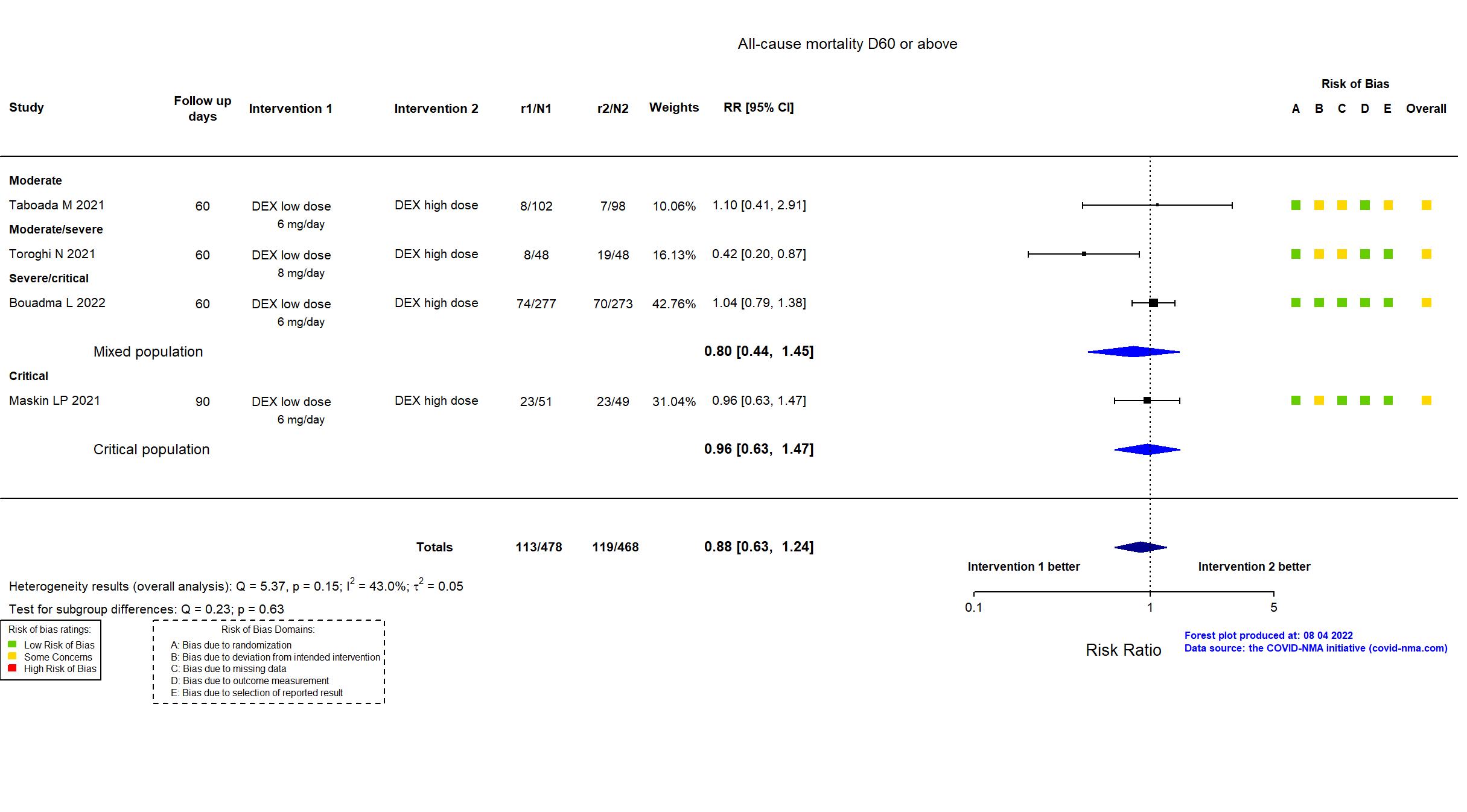


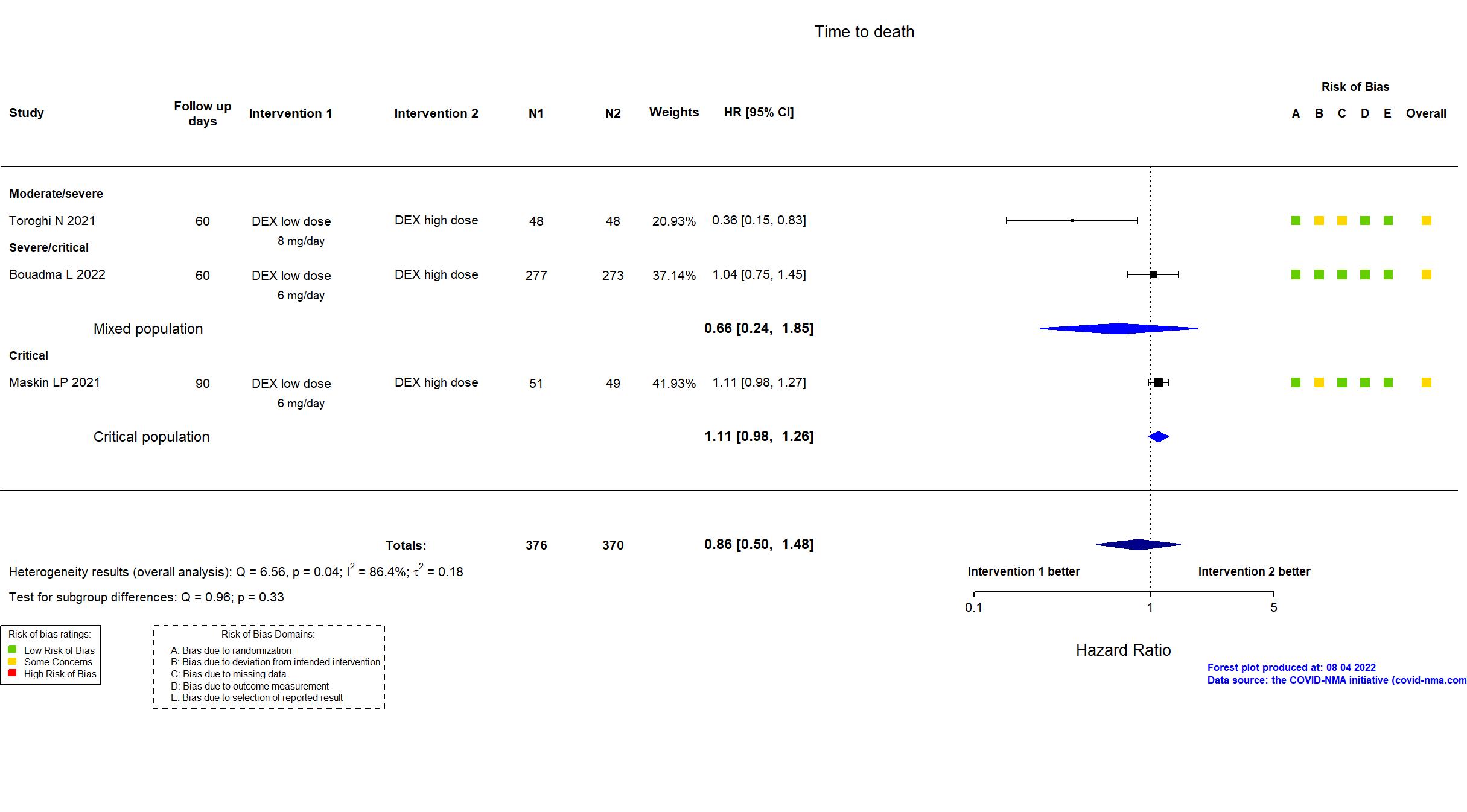
Studies included but not extracted/included in the analysis: Wu H, PloS one, 2022
FOREST PLOTS -2022-08-04






Trial NCT04344730 ; EudraCT 2020-001457-43
Publication COVIDICUS - Bouadma L, JAMA Intern Med (2022) (published paper)
Dates: 2020-04-10 to 2020-11-30
Funding: Mixed (Programme Hospitalier de Recherche Clinique, French Ministry of Health ; Vygon provided continuous positive airway pressure kits for some study centers.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: quadruple blinding | |
| Location :
Multicenter / France Follow-up duration (days): 60 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
DEX low dose 6 mg/day intravenously for 100 days (or placebo prior to RECOVERY trial results communication) |
|
| Control
DEX high dose 20 mg/day intravenously on days 1-5, then 10 mg/day intravenously on days 6-10 | |
| Participants | |
| Randomized participants : DEX low dose=277 DEX high dose=273 | |
| Characteristics of participants N= 550 Mean age : NR 414 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=448 Critical: n=98 | |
| Primary outcome | |
| In the register The time-to-death from all causes [Time Frame: day-60] ; The time to need for mechanical ventilation (MV) [Time Frame: day-28.] | |
| In the report Time-to-death from all causes up to day 60 ; time to IMV criteria fulfillment within the first 28 days | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the trial registry, protocol, statistical analysis plan and supplementary appendices were used in data extraction and assessment of risk of bias. This is a factorial trial design with a 2x3 randomization for a 2nd intervention as well based on type of oxygen therapy intervention. The primary and secondary outcomes in the article reflect those in the registry. The trial (n = 550) achieved its target sample size (n = 550). 37/276 participants in the low-dose arm were randomized prior to a protocol amendment and did not receive dexamethasone as a trial intervention because the pre-amendment comparison was high-dose dexamethasone versus placebo. |
Trial NCT04395105
Publication Maskin LP, Intensive Care Med (2021) (published paper)
Dates: 2020-06-17 to 2021-03-27
Funding: No specific funding (The author(s) received no financial support for the research, authorship
and/or publication of this article.)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Argentina Follow-up duration (days): 90 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
DEX low dose 6 mg intravenously once a day for 10 days |
|
| Control
DEX high dose 16 mg intravenously once a day for 5 days, then 8 mg intravenously once a day for 5 days | |
| Participants | |
| Randomized participants : DEX low dose=51 DEX high dose=49 | |
| Characteristics of participants N= 100 Mean age : NR 69 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=0 Critical: n=100 | |
| Primary outcome | |
| In the register 1) Ventilator-free days at 28 days [ Time Frame: 28 days after randomization ]; 2) Time to successful discontinuation from mechanical ventilation [ Time Frame: 28 days after randomization ] | |
| In the report 1) Ventilator-free days during the first 28 days; 2) Time to complete and successful discontinuation of mechanical ventilation or death. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published report, the pre-print article, the trial registry, protocol and supplementary appendices were used in data extraction and assessment of risk of bias. No statistical analysis plan was available. The primary outcomes in the article reflect those in the registry. The trial (n = 100) was terminated early due to changes in COVID-19 treatment modalities that led to reduced recruitment, so that it did not achieve its target sample size (n = 282).
The study was updated on the March 2nd, 2022 with data extracted from the published report. |
Trial *
Publication Montalvan E, Chest (2021) (published paper)
Dates: 2020-09-01 to 2020-10-31
Funding: Not reported/unclear
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / Honduras Follow-up duration (days): * | |
| Inclusion criteria |
|
| Exclusion criteria | NR |
| Interventions | |
| Treatment
DEX low dose 8 mg/day |
|
| Control
DEX high dose 24 mg/day | |
| Participants | |
| Randomized participants : DEX high dose=41 DEX low dose=40 | |
| Characteristics of participants N= 81 Mean age : NR 46 males Severity : Mild: n=* / Moderate: n=* / Severe: n=* Critical: n=* | |
| Primary outcome | |
| In the register NR | |
| In the report Reduction in mortality and intubation. | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
* |
| General comment | Only the conference abstract was available for use in data extraction and assessment of risk of bias. No registry, protocol or statistical analysis plan was available. Mortality was reported (6 in the high-dose group and 2 in the low-dose group), but with no time point reported, so no data were extracted. |
Trial NCT04726098 ; EudraCT 2020-005702-25
Publication Taboada M, Eur Respir J (2021) (published paper)
Dates: 2021-01-15 to 2021-05-26
Funding: Public/non profit (Support was provided solely from institutional and departmental sources.)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / Spain Follow-up duration (days): 60 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
DEX low dose 6 mg once daily for 10 days |
|
| Control
DEX high dose 20 mg once daily for 5 days, followed by 10 mg once daily for 5 days | |
| Participants | |
| Randomized participants : DEX high dose=98 DEX low dose=102 | |
| Characteristics of participants N= 200 Mean age : NR 123 males Severity : Mild: n=0 / Moderate: n=200 / Severe: n=0 Critical: n=0 | |
| Primary outcome | |
| In the register Percentage of patients with treatment failure at day 11 [ Time Frame: Day 11 after randomization ] defined as death, need of ICU and extracorporeal membrane oxygenation, need of non-invasive ventilation or nasal high-flow oxygen therapy, or worsening of the condition clinic of the patient during treatment (two of these: need to increase Fraction of inspired oxygen inspired>20%, need for fraction inspired oxygenation>50%, increase in respiratory rate>25, increase in inflammatory markers). | |
| In the report Clinical worsening within 11 days since randomisation, defined as worsening of the patient’s condition during treatment (need to increase fraction of inspired oxygen>0.2, need for fraction of inspired oxygen>0.5, respiratory rate>25) or score higher than 4 on the 7-point ordinal scale WHO-CIS. | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the trial registry and supplementary appendices were used in data extraction. Neither protocol nor statistical analysis plan was available. There were minor differences between the primary outcomes in the registry and the article and the extracted 28-day and 60-day outcome were not included in the registry. The study (n = 200) achieved its target sample size (n = 196). |
Trial IRCT20100228003449N31
Publication Toroghi N, Pharmacol Rep (2021) (published paper)
Dates: 2020-10-26 to 2021-01-15
Funding: Public/non profit (Tehran University of Medical Sciences)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / Iran Follow-up duration (days): 60 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
DEX low dose 8 mg orally once a day for up to 10 days or discharge DEX intermediate 8 mg orally twice a day for up to 10 days or discharge |
|
| Control
DEX high dose 8 mg orally three times a day for up to 10 days or discharge | |
| Participants | |
| Randomized participants : DEX low dose=48 DEX intermediate=48 DEX high dose=48 | |
| Characteristics of participants N= 144 Mean age : NR 80 males Severity : Mild: n=0 / Moderate: n=* / Severe: n=* Critical: n=0 | |
| Primary outcome | |
| In the register 1) Improvement of patients' chief complaint 2) Improvement in peripheral blood oxygen saturation 3) Decrease in serum CRP 4) Hyperglycemia 5) Changes in mood 6) Myopathy 7) Secondary infections | |
| In the report Time to a clinical response that was described as improvement of at least two scores in the eight-category ordinal scale of the National Institute of Health | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the trial registry and supplementary materials were used in data extraction and assessment of risk of bias. Neither protocol nor statistical analysis plan was available. The primary outcome in the published article (an improvement of two or more points on an 8-point ordinal scale) broadly reflects one of the outcomes in the registry (improvement of patient’s chief complaint). Other primary outcomes in the registry were reported as secondary outcomes. The trial (n = 144) achieved its target sample size (n = 144). |