Molnupiravir vs Standard care/Placebo (RCT)
Hospitalized patients
FOREST PLOTS -2022-09-15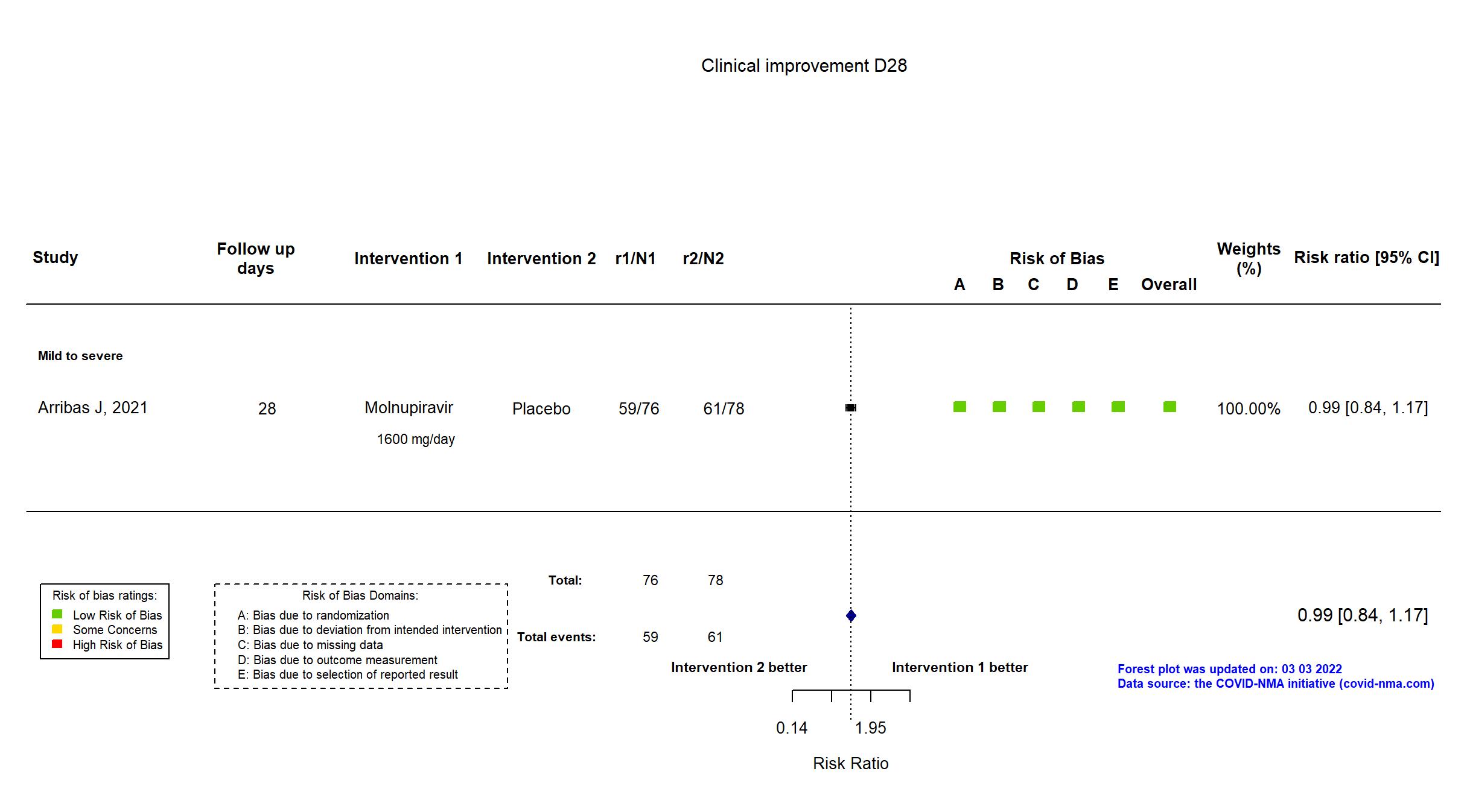
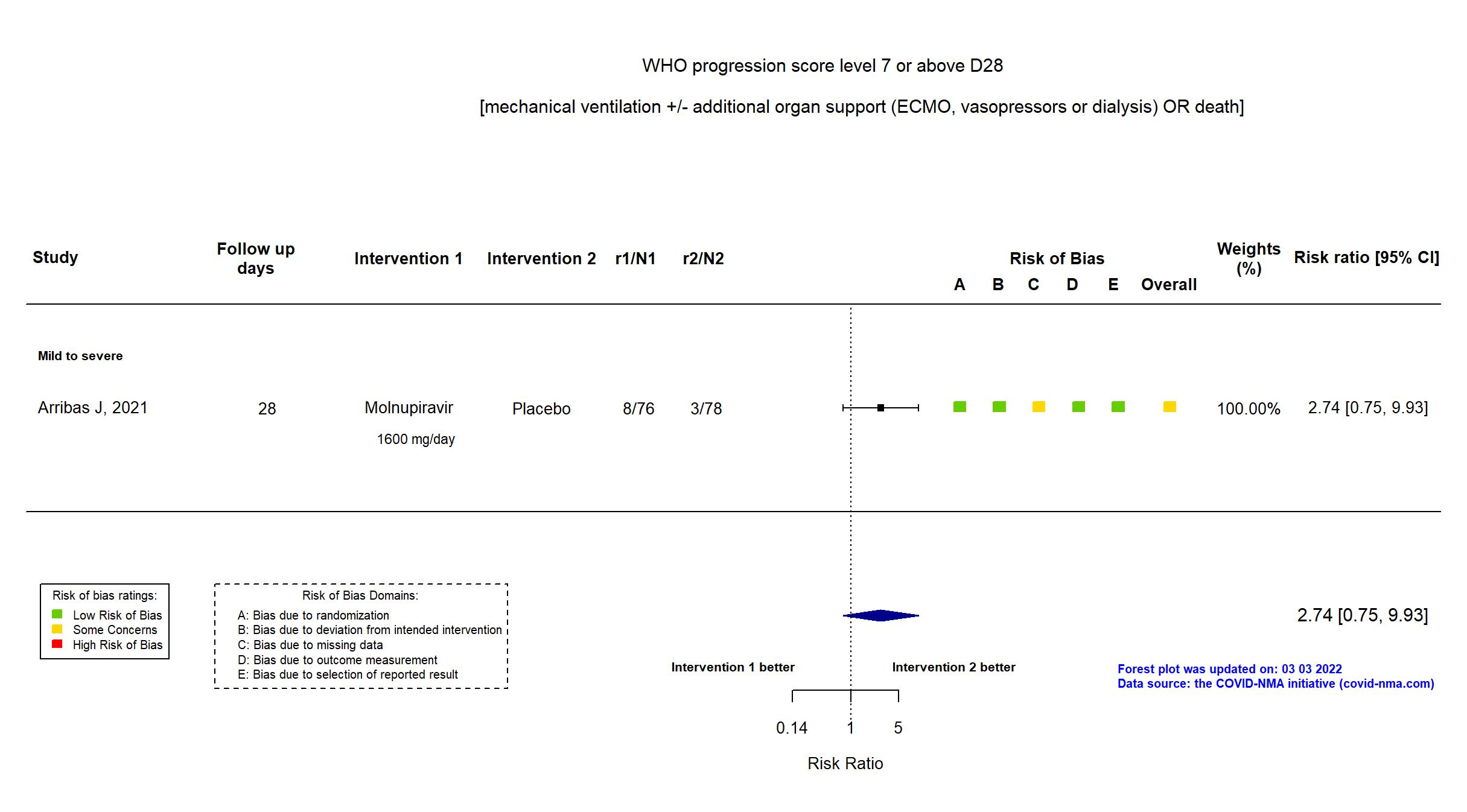
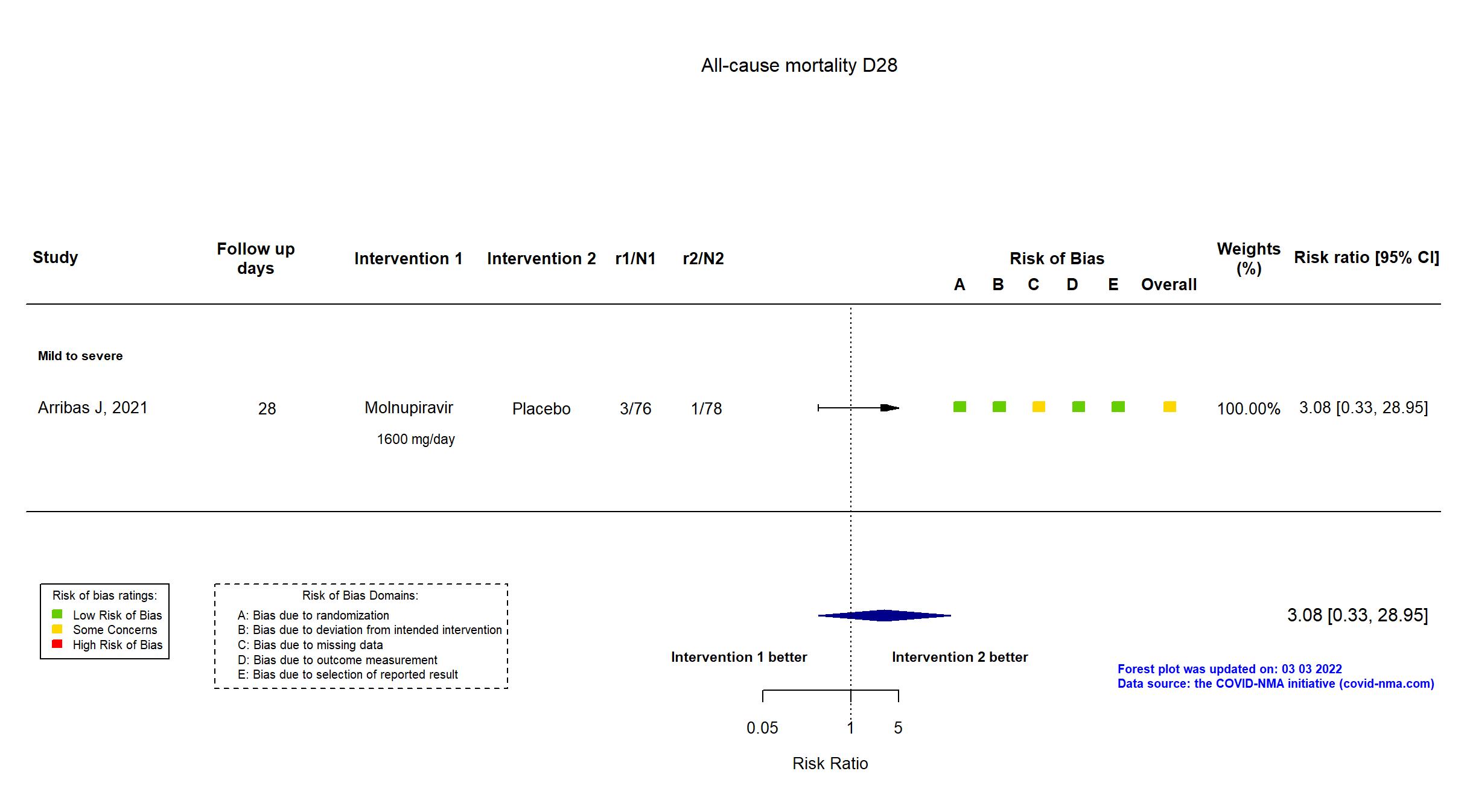
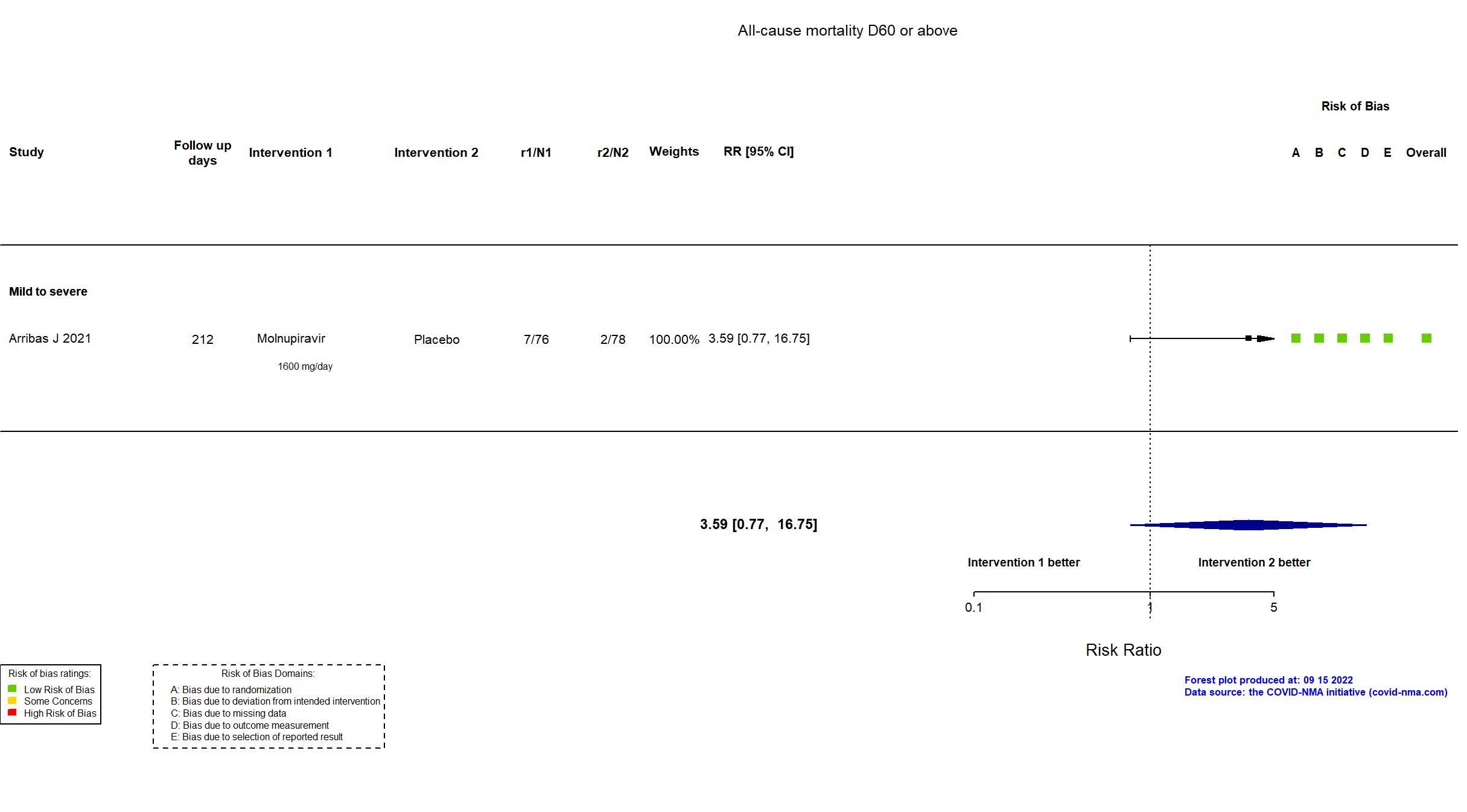
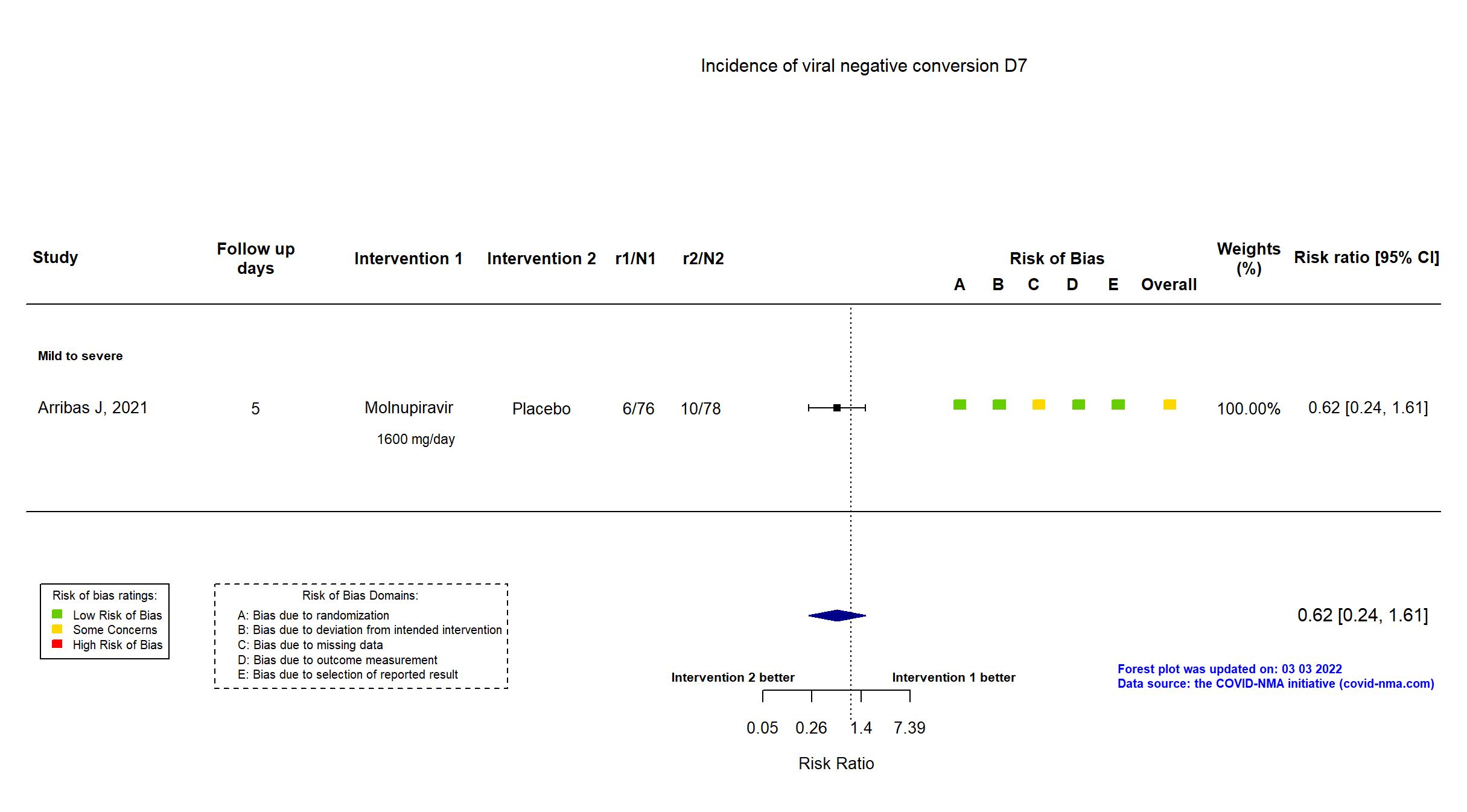
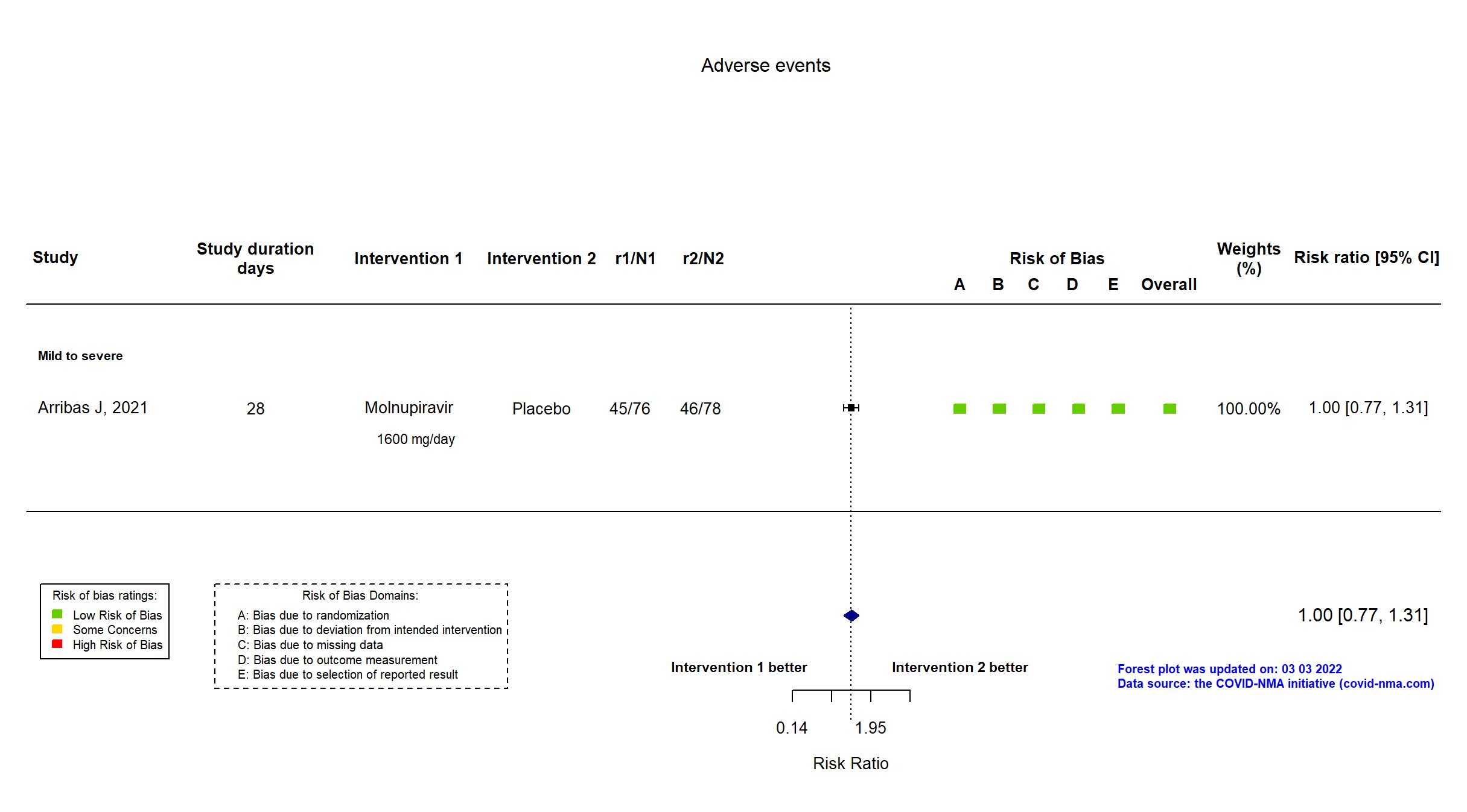
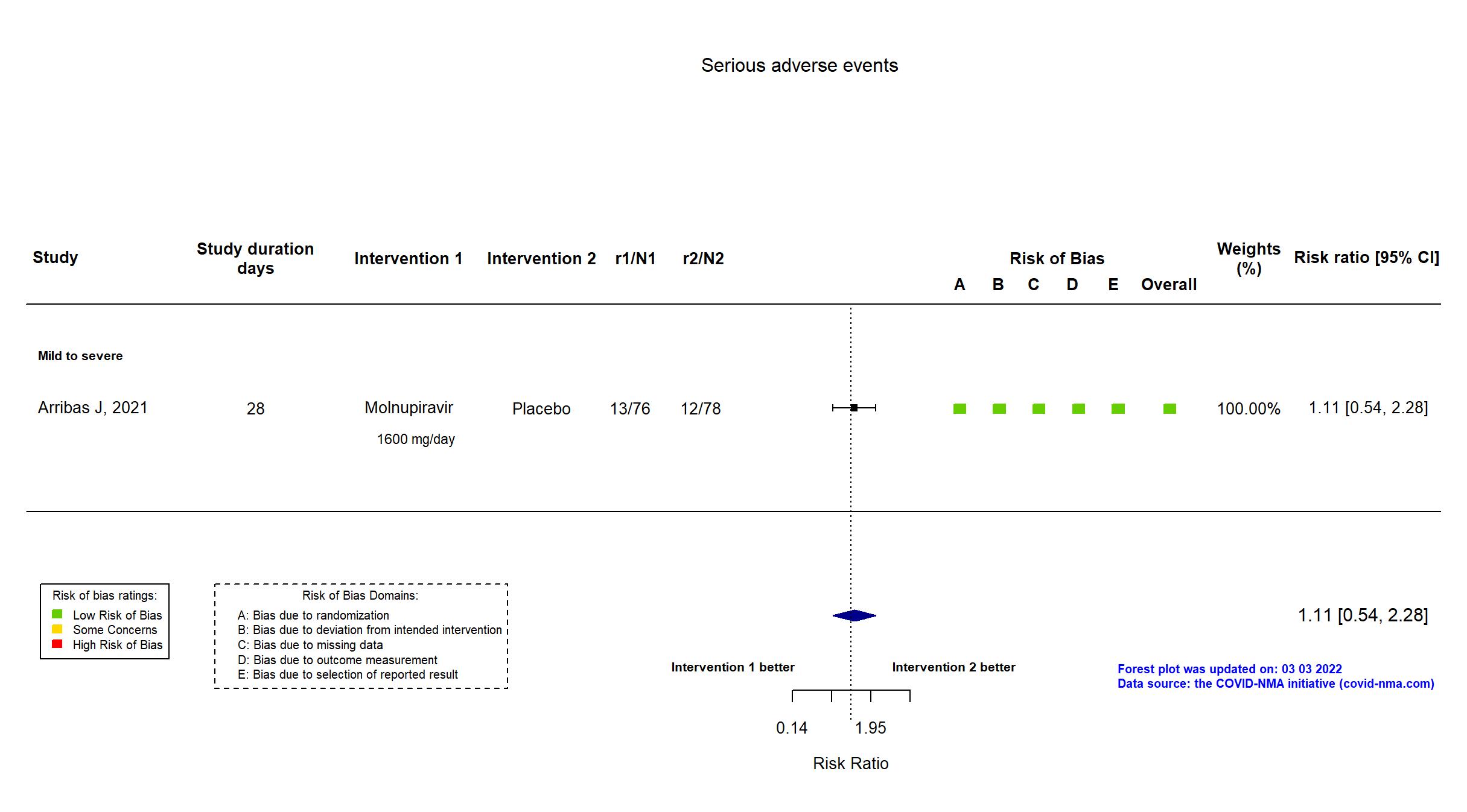
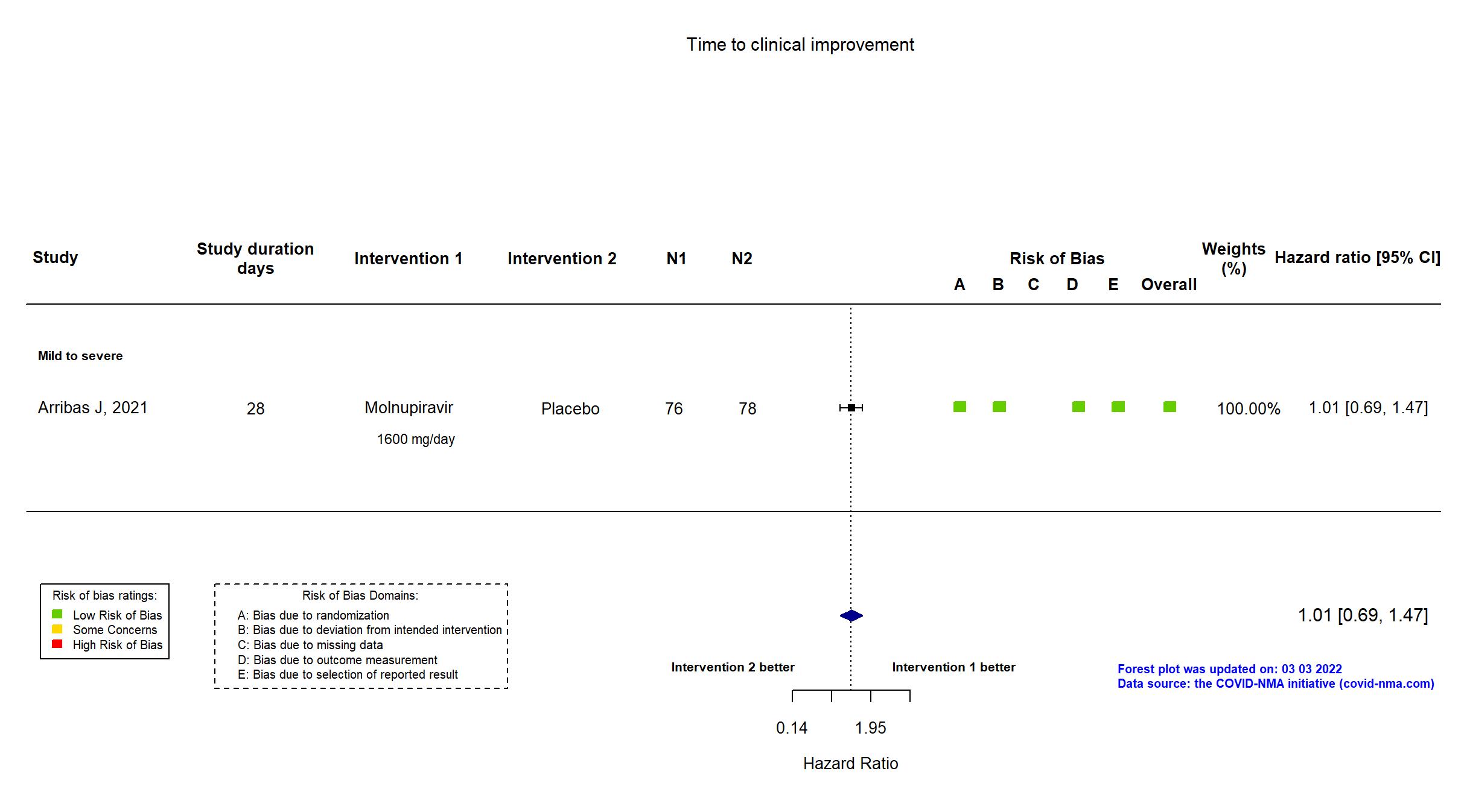








Trial NCT04575584
Publication MOVe-IN - Arribas J, N Engl J Med Evidence (2021) (published paper)
Dates: 2020-10-19 to 2021-01-12
Funding: Private (Merck Sharp & Dohme Corp.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / Brazil, Chile, Colombia, France, Israel, Mexico, Philippines, Poland, Russian Federation, South Africa, Republic of Korea, Spain, UK, Ukraine, USA Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Molnupiravir 200mg 200 mg orally twice daily for 5 days Molnupiravir 400mg 400 mg orally twice daily for 5 days Molnupiravir 800 mg orally twice daily for 5 days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Molnupiravir 200mg=75 Molnupiravir 400mg=75 Molnupiravir=76 Placebo=78 | |
| Characteristics of participants N= 304 Mean age : NR 172 males Severity : Mild: n=148 / Moderate: n=134 / Severe: n=11 Critical: n=0 | |
| Primary outcome | |
| In the register 1) Time-to-sustained recovery [ Time Frame: Up to 29 days ]; 2) Percentage of participants with an adverse event (AE) [ Time Frame: Up to 7 months ]; 3) Percentage of participants who discontinued study intervention due to an AE [ Time Frame: Up to 6 days ] | |
| In the report Safety and sustained recovery: 1) Rates of adverse events reported during the safety follow-up period (from randomization through 14 days after end of treatment); 2) Adverse events leading to discontinuation of study intervention reported during the safety follow-up period; 3) Sustained recovery, defined as participants being alive and either not hospitalized or medically ready for hospital discharge through day 29 | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published article, the registry, protocol, statistical analysis plan and supplementary appendices were used in data extraction and assessment of risk of bias. There were no major differences between the registry and the article in populations, procedures, interventions or outcomes: the primary outcomes in the article reflected those in the registry, but different timepoints are reported for adverse events. Recruitment was terminated for commercial reasons and so the study (n = 304) did not achieve its target sample size (n = 1300).
This study was updated on September 14th, 2022 with data extracted from the registry. |