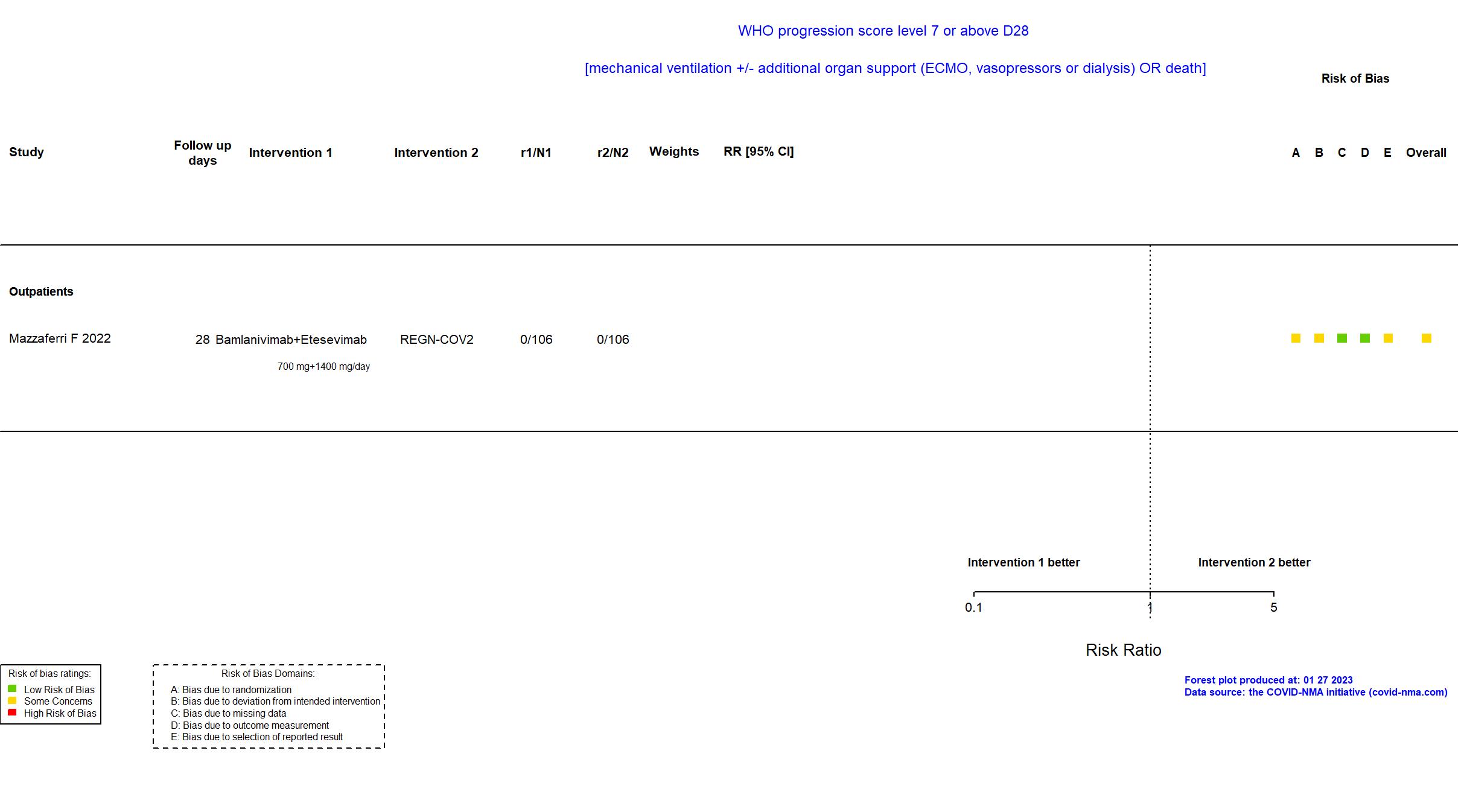
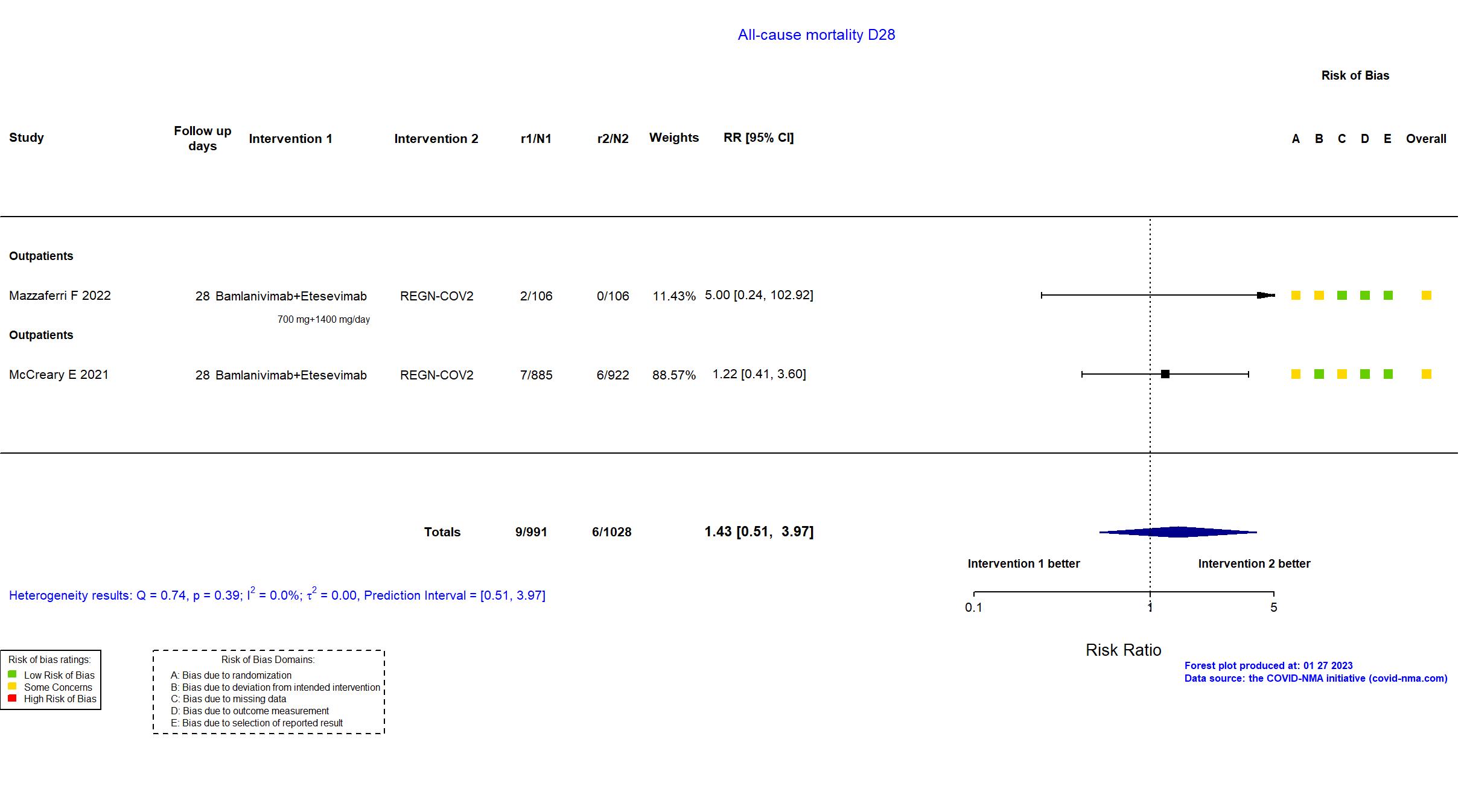
Bamlanivimab+Etesevimab vs REGN-COV2 (RCT)
Mild outpatients
FOREST PLOTS -2023-01-27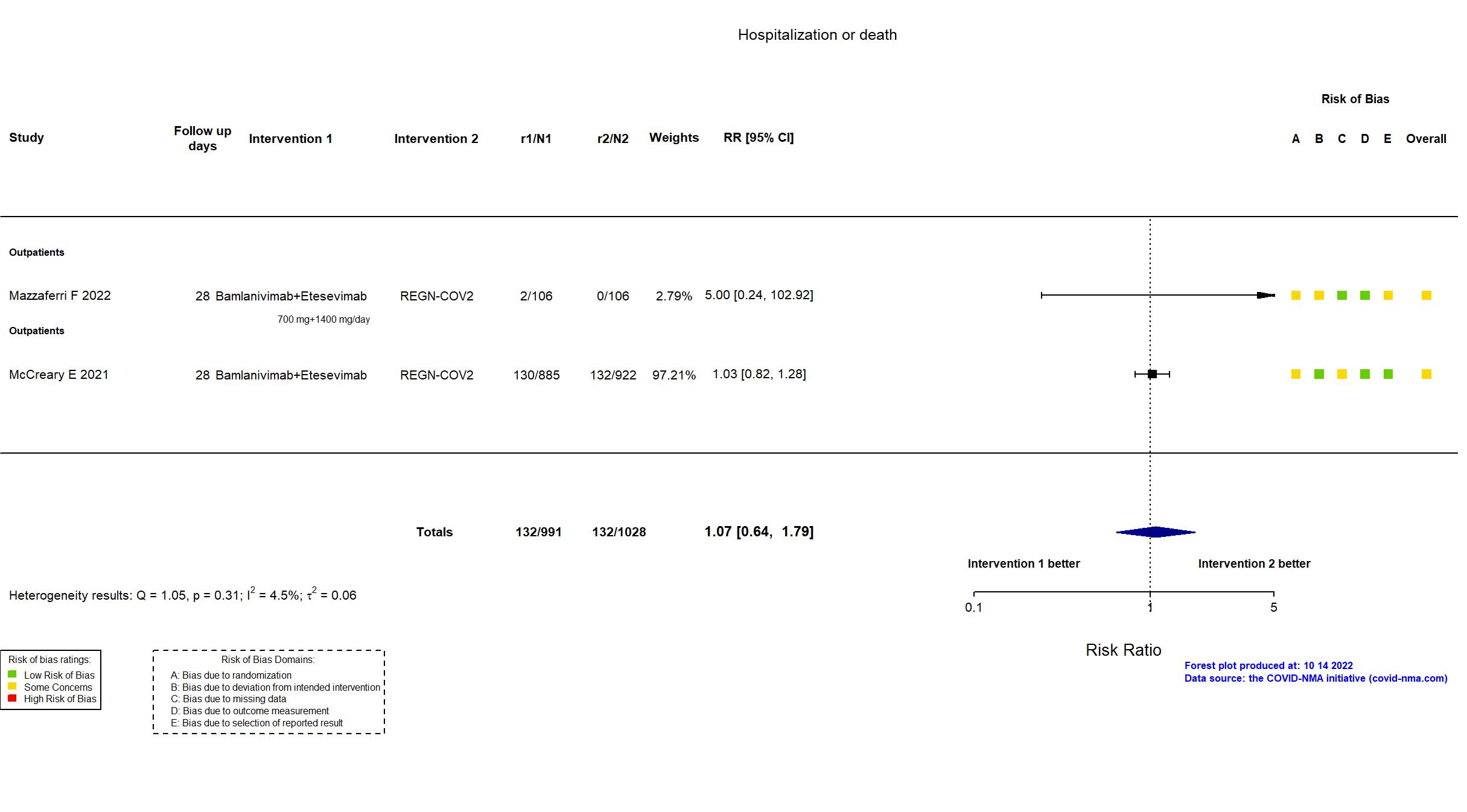



Trial NCT05205759
Publication MANTICO - Mazzaferri F, eLife (2022) (published paper)
Dates: 2021-12-09 to 2022-01-20
Funding: Public/non profit (Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA); the ORCHESTRA (Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic) project, which has received funding from the European Union’s Horizon 2020 research and innovation programme)
Conflict of interest: No
| Methods | |
| RCT Blinding: single blinding | |
| Location :
Multicenter / Italy Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Sotrovimab 500 mg of sotrovimab by intravenous infusion single dose Bamlanivimab+Etesevimab 700 mg of bamlanivimab + 1400 mg of etesevimab by intravenous infusion single dose |
|
| Control
REGN-COV2 600 mg of casirivimab + 600 mg of imdevimab by intravenous infusion single dose | |
| Participants | |
| Randomized participants : REGN-COV2=106 Sotrovimab=107 Bamlanivimab+Etesevimab=106 | |
| Characteristics of participants N= 319 Mean age : NR 170 males Severity : Mild: n= 311/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register COVID-19 progression [ Time Frame: 14 days ] (1) hospitalization or (2) need of supplemental oxygen therapy at home or (3) death within 14 days of randomisation | |
| In the report COVID-19 progression, defined as hospitalisation, need of supplemental oxygen therapy, or death from any cause through day 14. | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published article, the pre-print article, the trial registry was used in data extraction and assessment of risk of bias. There is no change from the trial registration in the intervention and control treatments. The primary outcome in the article reflects that in the registry. Results are reported per variant of concerns (delta and omicron). Adverse events are not reported. The trial (n = 319) did not achieve its target sample size (n = 1260) as recruitment was interrupted after the publication of in-vitro evidence that two treatments under investigation (bamlanivimab/etesevimab and casirivimab/imdevimab) were not effective against the new emerging viral Omicron VOC.
This study was updated on January 19th, 2023 with data extracted from the published report. |
Trial NCT04790786
Publication OPTIMISE-C19 - McCreary E, medRxiv (2021) (preprint)
Dates: 2021-03-10 to 2021-06-25
Funding: Public/non profit (Internal funding from the UPMC Hospital System. The U.S. federal government provided the monoclonal antibody treatments reported in this manuscript.)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / USA Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Bamlanivimab 700 mg intravenously once-off Bamlanivimab+Etesevimab |
|
| Control
REGN-COV2 1200 mg + 1200 mg intravenously once-off | |
| Participants | |
| Randomized NR Analyzed 1935 participants Bamlanivimab =128 Bamlanivimab+Etesevimab=885 REGN-COV2=922 | |
| Characteristics of participants N= 1935 Mean age : NR 894 males Severity : Mild: n= 1935/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register Alive and Free from Hospitalization [ Time Frame: 28 days after initial participation ] | |
| In the report Hospital-free days up to day 28 after mAb treatment | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: N |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the pre-print article, the trial protocol, statistical analysis plan, registry and supplementary materials were used in data extraction and assessment of risk of bias. The primary outcome reported in the paper reflects the primary outcome indicated in registry and protocol. Ten laboratory and immunological outcomes in the registry were not reported. The study (n = 2466) did not achieve its target sample size (n = 5000) at time of reporting because two of the three study treatments were withdrawn, so that patients could no longer be randomized to these. The U.S. Department of Health and Human Services halted distribution of bamlanivimab alone on March 25, 2021, and of bamlanivimab-etesevimab on June 25, 2021, due to concern of lack of efficacy against certain SARS-CoV-2 variants that were predominant in the U.S. at those times. Recruitment continues to the Casirivimab + Imdevimab and Sotrovimab arms. |