Studies description
Trial NCT04464408
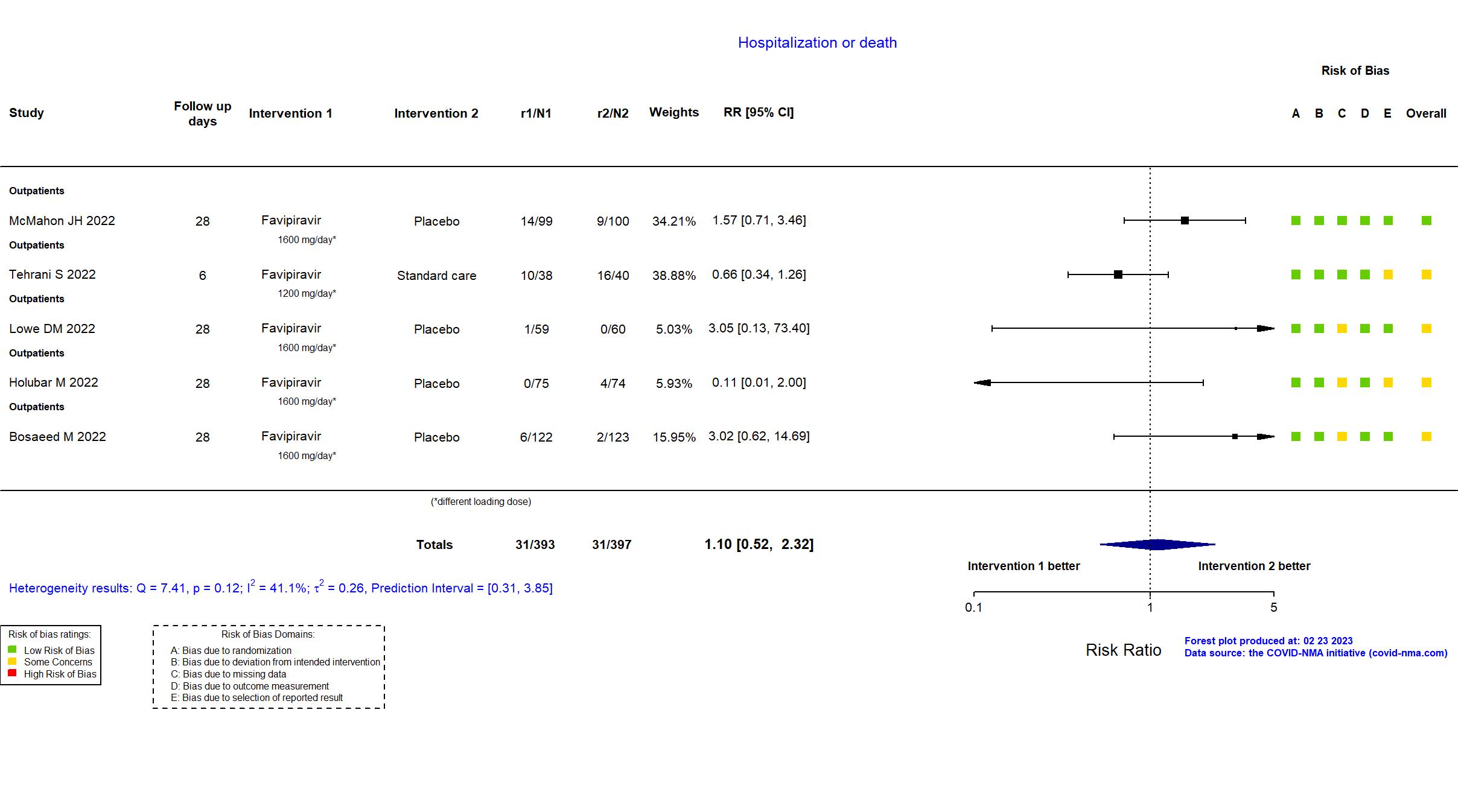
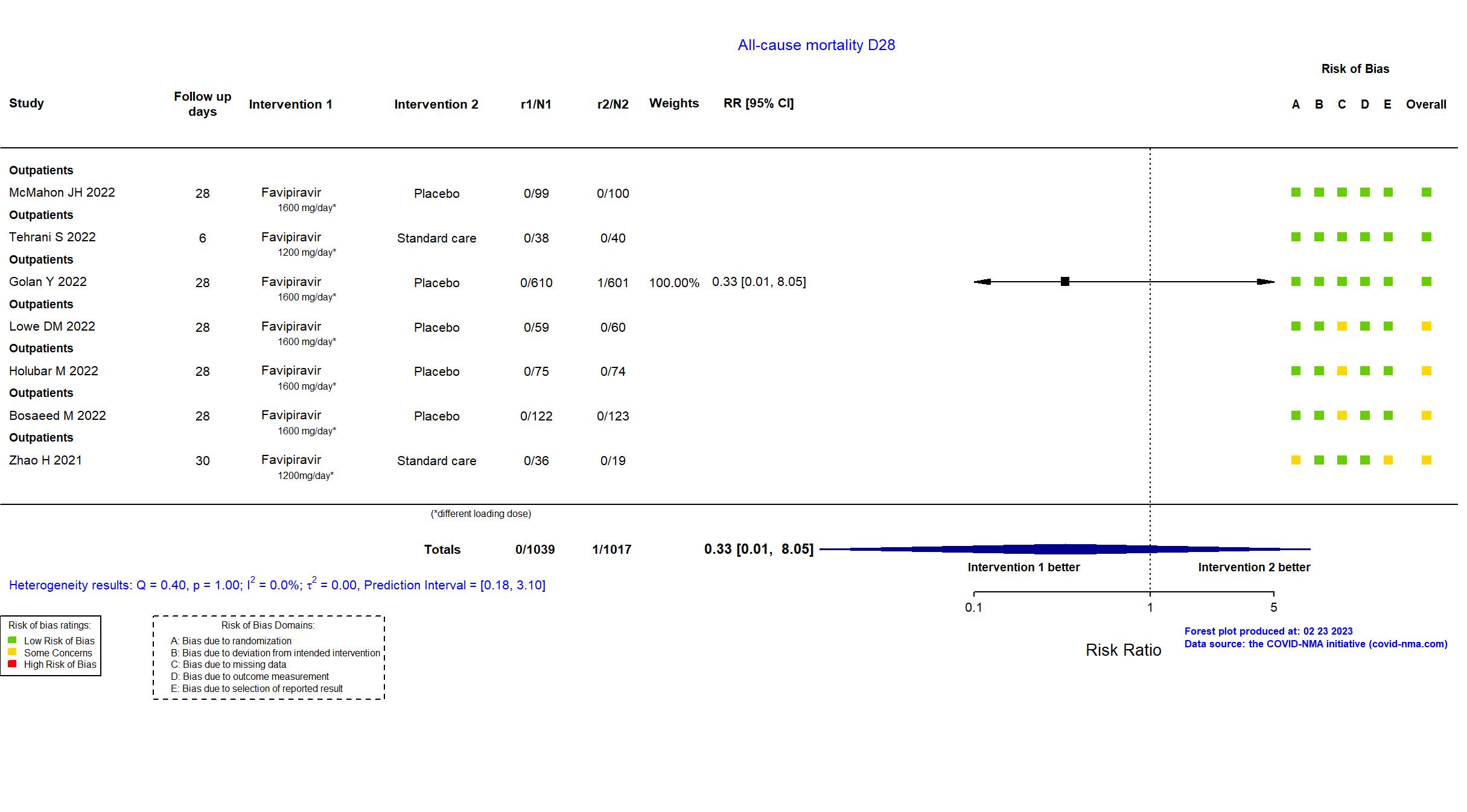
Publication Bosaeed M, Clin Microbiol Infect (2022) (published paper)
Dates: 2020-07-23 to 2021-08-04
Funding: Public/non profit (King Abdullah International Medical Research Center (KAIMRC).)
Conflict of interest: No
Trial NCT04600895
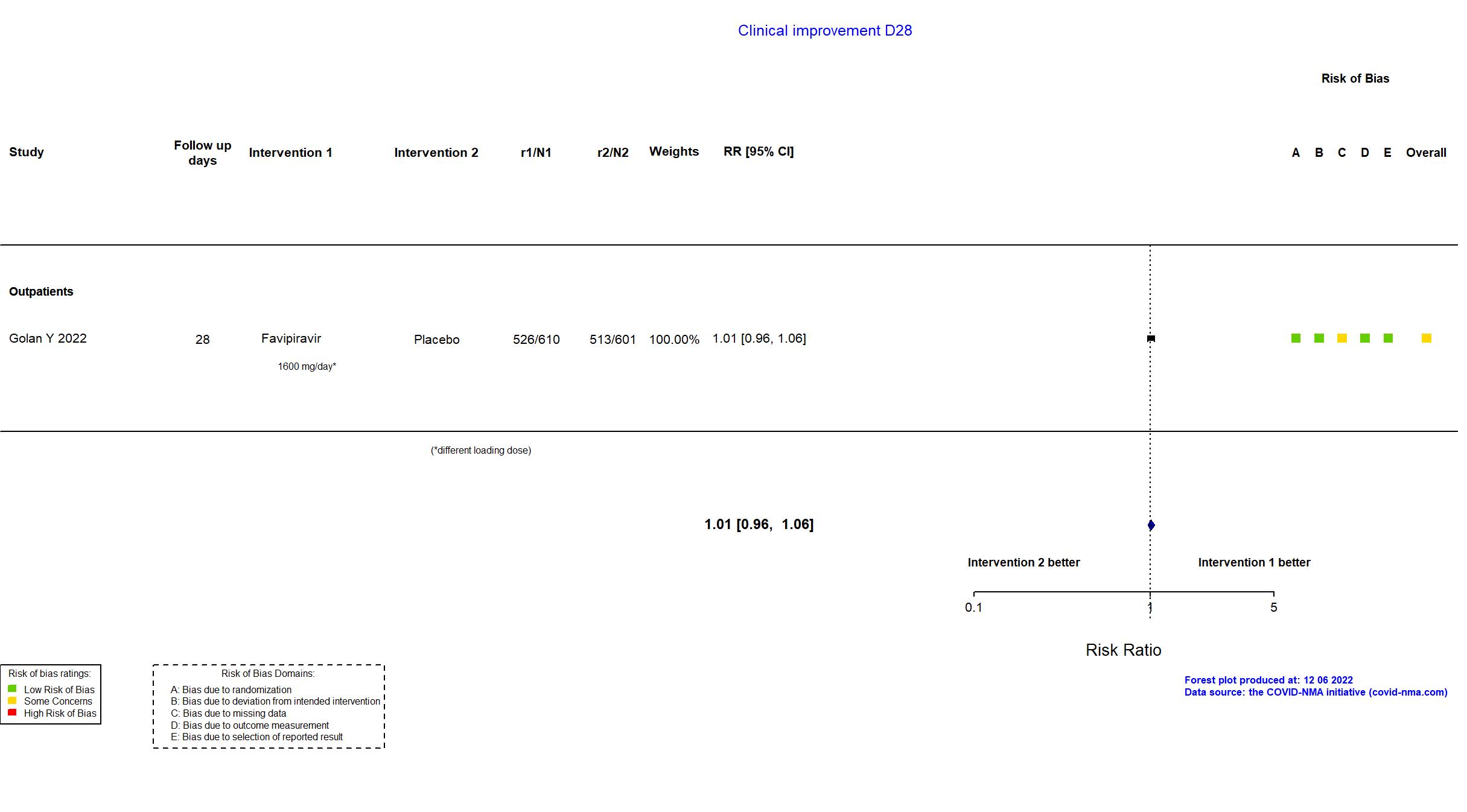
Publication PRESECO - Golan Y, Clin Infect Dis (2022) (published paper)
Dates: 2020-11-30 to 2021-10-20
Funding: Private (The conduct of this clinical trial and the preparation
of this manuscript were supported by Appili Therapeutics, Inc., Halifax,
NS, Canada.)
Conflict of interest: Yes
Trial NCT04346628
Publication Holubar M, Clin Infect Dis (2022) (published paper)
Dates: 2020-07-08 to 2021-03-23
Funding: Public/non profit (Anonymous donors to Stanford University)
Conflict of interest: No
Trial NCT04499677
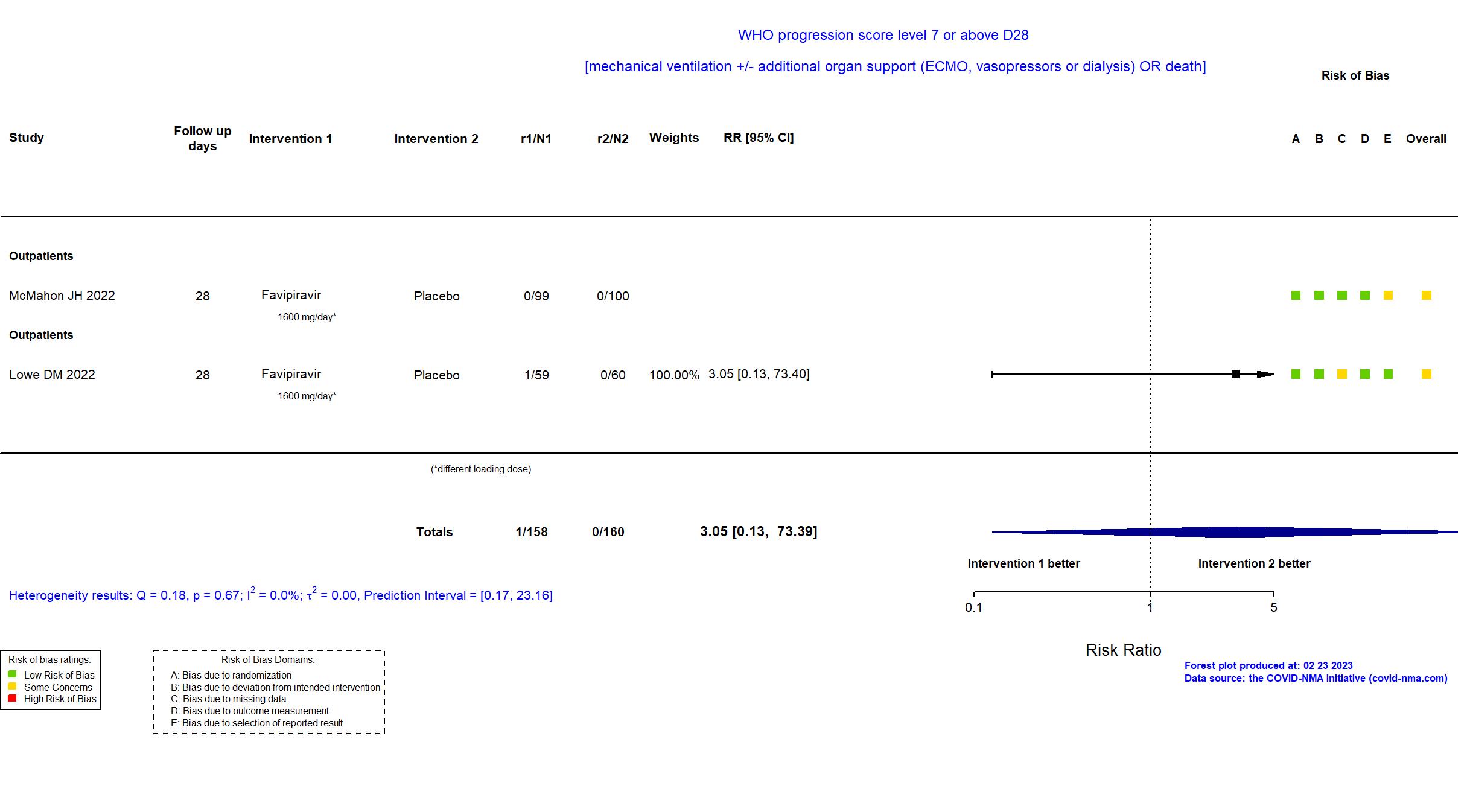
Publication FLARE - Lowe DM, PLoS Med (2022) (published paper)
Dates: 2020-10-06 to 2021-11-04
Funding: Mixed (LifeArc, UK; Fujifilm Toyama Chemical Co. provided favipiravir and favipiravir placebo free of charge.)
Conflict of interest: Yes
Trial NCT04445467
Publication VIRCO - McMahon JH, EClinicalMedicine (2022) (published paper)
Dates: 2020-07-31 to 2021-09-19
Funding: Mixed (The study was supported in part by grants from the Commonwealth Bank Australia, the Lord Mayor’s Charitable Foundation, Melbourne Australia and the Orloff Family Charitable Trust, Melbourne, Australia. JHM is supported by the Medical Research Future Fund, AYP, JT are supported by the Australian National Health and Medical Research Council.)
Conflict of interest: No
Trial IRCT20211004052664N1
Publication Tehrani S, Mediterr J Infect Microbes Antimicrob (2022) (published paper)
Dates: 2021-04-01 to 2021-09-30
Funding: No specific funding (The authors declared that this study received no financial support.)
Conflict of interest: No
Trial NCT04333589
Publication Zhao H, Int Immunopharmacol (2021) (published paper)
Dates: 2020-03-27 to 2020-05-09
Funding: Public/non profit (Chinese COVID-19 scientific research emergency; China Mega-Project for Infectious Diseases; China Mega-Project for Innovative Drugs)
Conflict of interest: No