Studies description
Trial NCT04694612
Publication Adhikari P, Int J Infect Dis (2022)
Funding: Not reported/unclear
Conflict of interest: *
Trial NCT04387760
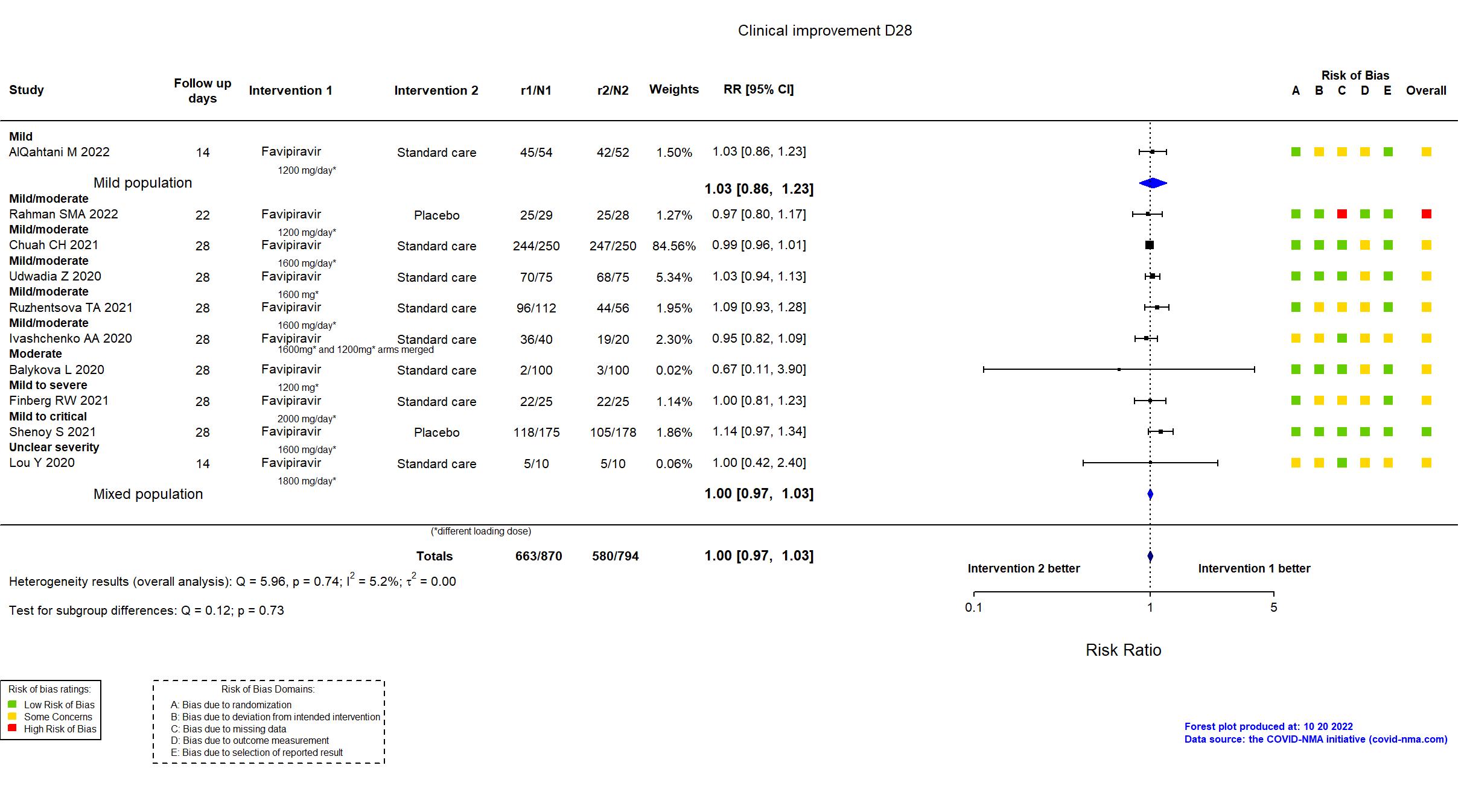
Publication AlQahtani M, Sci Rep (2022) (published paper)
Dates: 2020-08-01 to 2021-03-30
Funding: Public/non profit (National Taskforce for combating the Coronavirus (COVID- 19), Ministry of Health Bahrain; the Royal College of Surgeons in Ireland-Bahrain.)
Conflict of interest: No
Trial NCT04542694
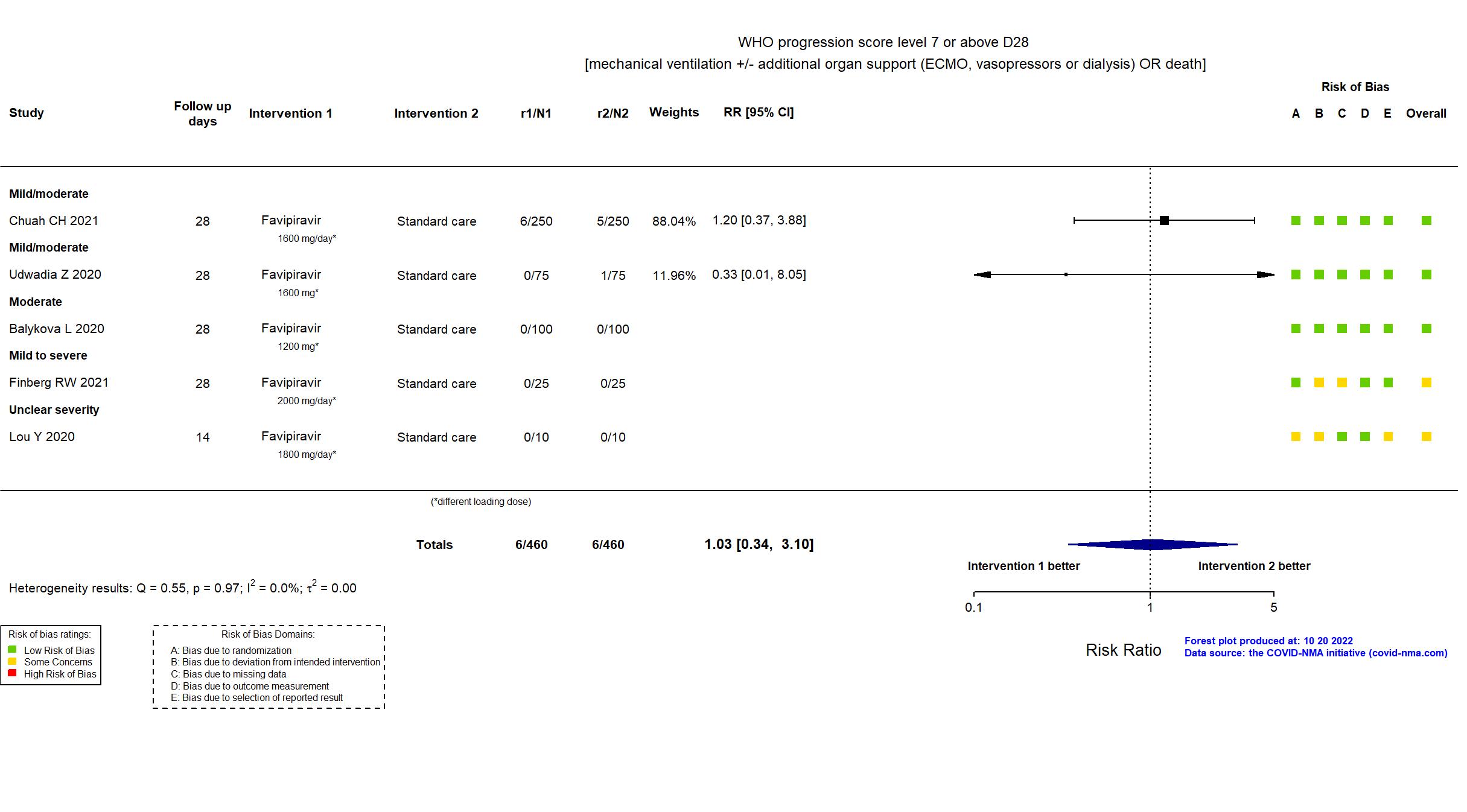
Publication Balykova L, Infektsionnye bolezn (2020) (published paper)
Funding: Private (Promomed, LLC)
Conflict of interest: No
Trial NCT04818320
Publication Chuah CH, Clin Infect Dis (2021) (published paper)
Dates: 2021-02-01 to 2021-06-20
Funding: No specific funding
Conflict of interest: No
Trial NCT04358549
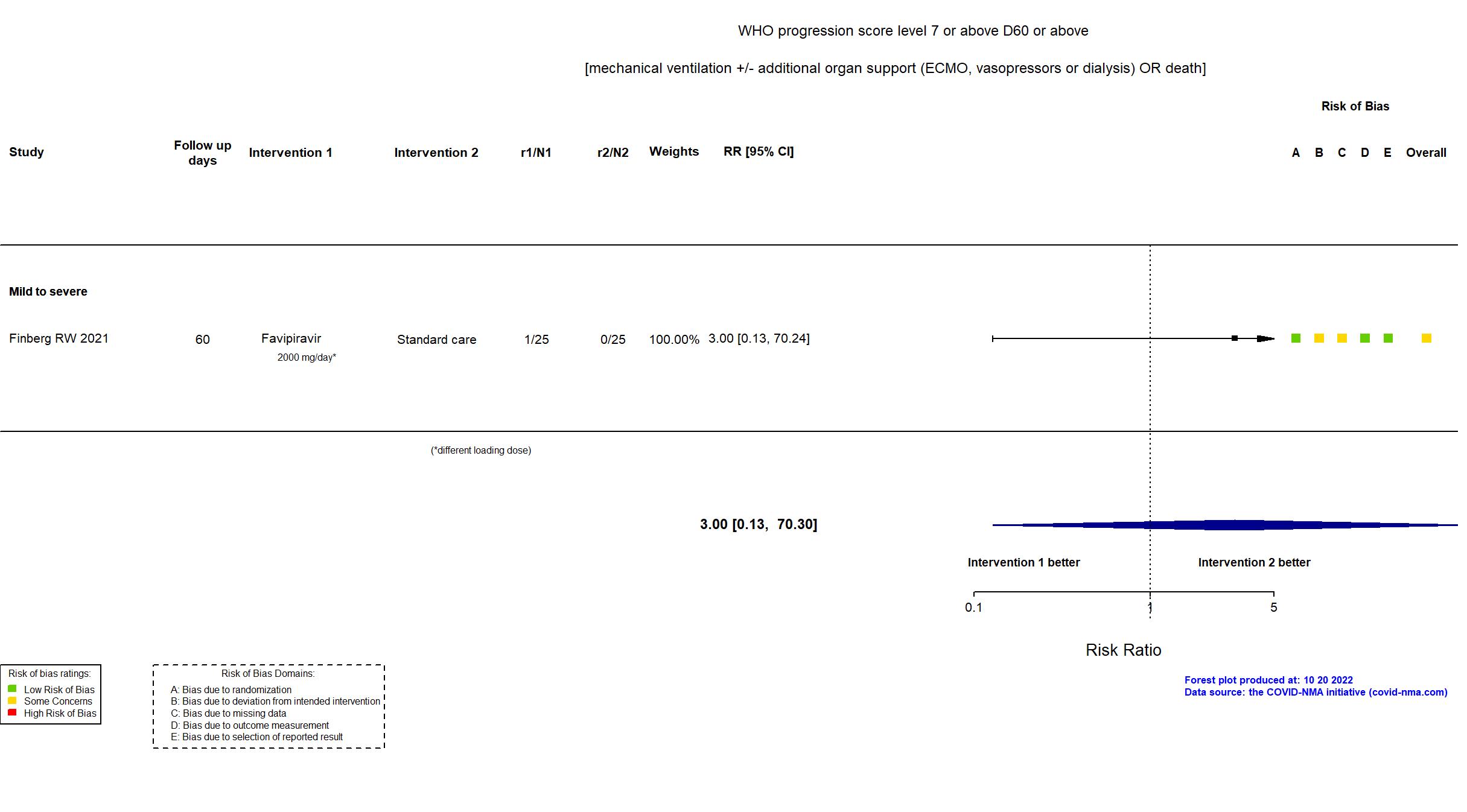
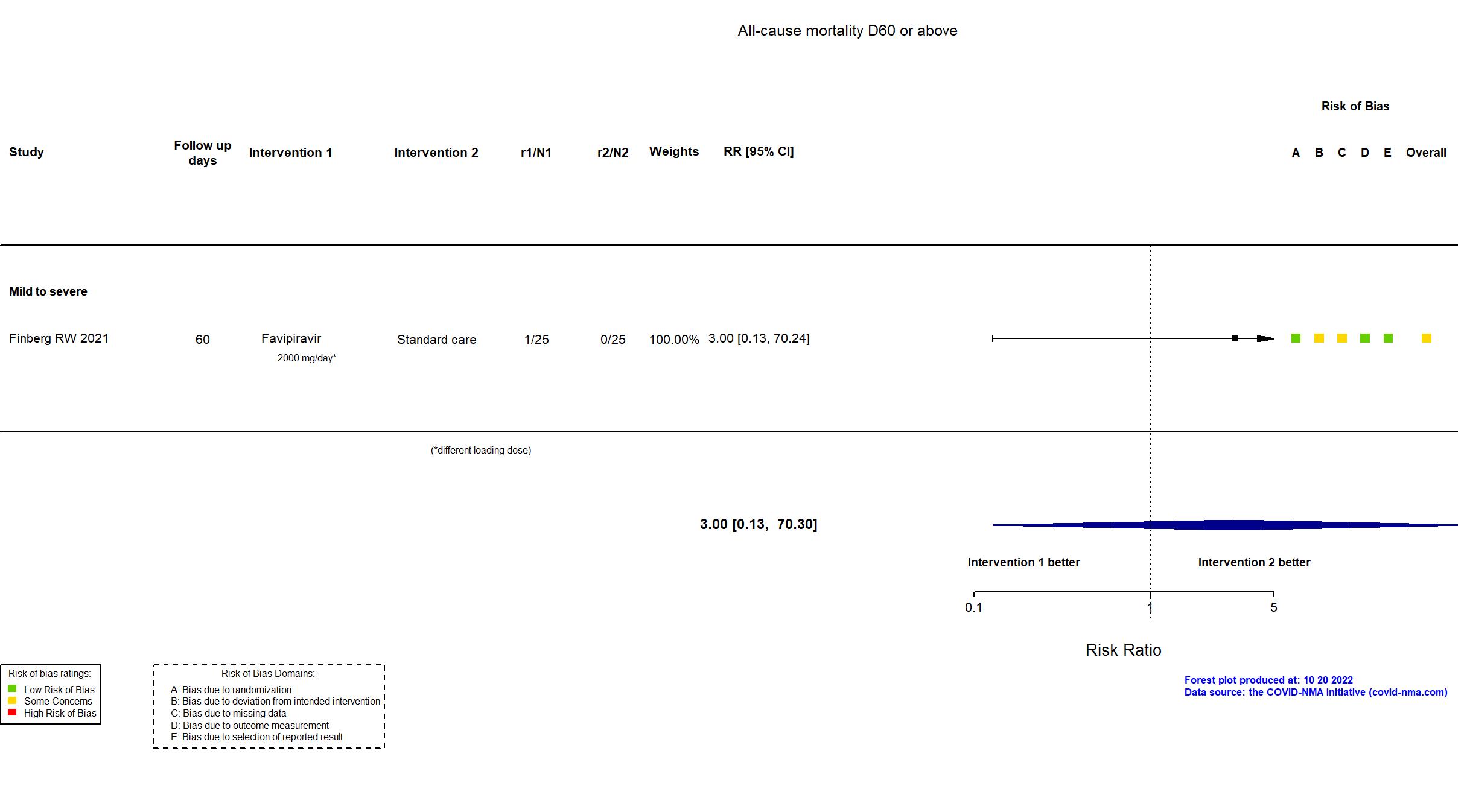
Publication Finberg RW, Open Forum Infect Dis (2021) (published paper)
Dates: 2020-04-17 to 2020-10-30
Funding: Mixed (US Army Medical Research and Development Command (USAMRDC); FUJIFULM Pharmaceuticals USA, Inc)
Conflict of interest: Yes
Trial NCT04434248
Publication Ivashchenko AA, Clin Infect Dis (2020) (published paper)
Funding: Mixed (Russian Direct Investment Fund, the Ministry of Industry and Trade of the Russian Federation, The Skolkovo Innovation Center. )
Conflict of interest: Yes
Trial ChiCTR2000029544
Publication Lou Y, Eur J Pharm Sci (2020) (published paper)
Funding: Public/non profit (Zhejiang Provincial Science and technology department key R & D plan emergency project)
Conflict of interest: No
Trial NCT04402203
Publication Rahman SMA, Clin Infect Pract (2022) (published paper)
Dates: 2020-05-01 to 2020-07-30
Funding: Private (Beacon Pharmaceuticals Limited, Dhaka, Bangladesh.)
Conflict of interest: No
Trial NCT04501783
Publication Ruzhentsova TA, Am J Transl Res (2021) (published paper)
Dates: 2020-05-23 to 2020-06-30
Funding: Private (R-Pharm Group of Companies)
Conflict of interest: No
Trial NCT04529499; IND148286
Publication Shenoy S, medRxiv (2021) (preprint)
Dates: 2020-08-22 to 2020-01-27
Funding: Private (Dr. Reddys Laboratories Ltd. and Global Response Aid )
Conflict of interest: Yes
Trial JapicCTI-205238
Publication Shinkai M, Infect Dis Ther (2021) (published paper)
Dates: 2020-04-02 to 2020-08-16
Funding: Private (FUJIFILM Toyama Chemical Co., Ltd. )
Conflict of interest: Yes
Trial TCTR20200514001
Publication Sirijatuphat R, Emerg Microbes Infect (2022) (published paper)
Dates: 2020-12-01 to 2021-07-30
Funding: Mixed (National Research Council of Thailand; Siriraj Research and Development Fund, Faculty of Medicine Siriraj Hospital, Mahidol University; Unitaid; Wellcome Trust; EPSRC; NIH. The study
drug (Favipiravir) was supported by FUJIFILM Toyama Chemical Co., Ltd.)
Conflict of interest: No
Trial CTRI/2020/05/025114
Publication Udwadia Z, Int J Infect Dis (2020) (published paper)
Funding: Private (Glenmark Pharmaceuticals Limited, India)
Conflict of interest: Yes