Tenofovir + Emtricitabine vs Standard care (RCT)
Hospitalized patients
FOREST PLOTS -2022-11-18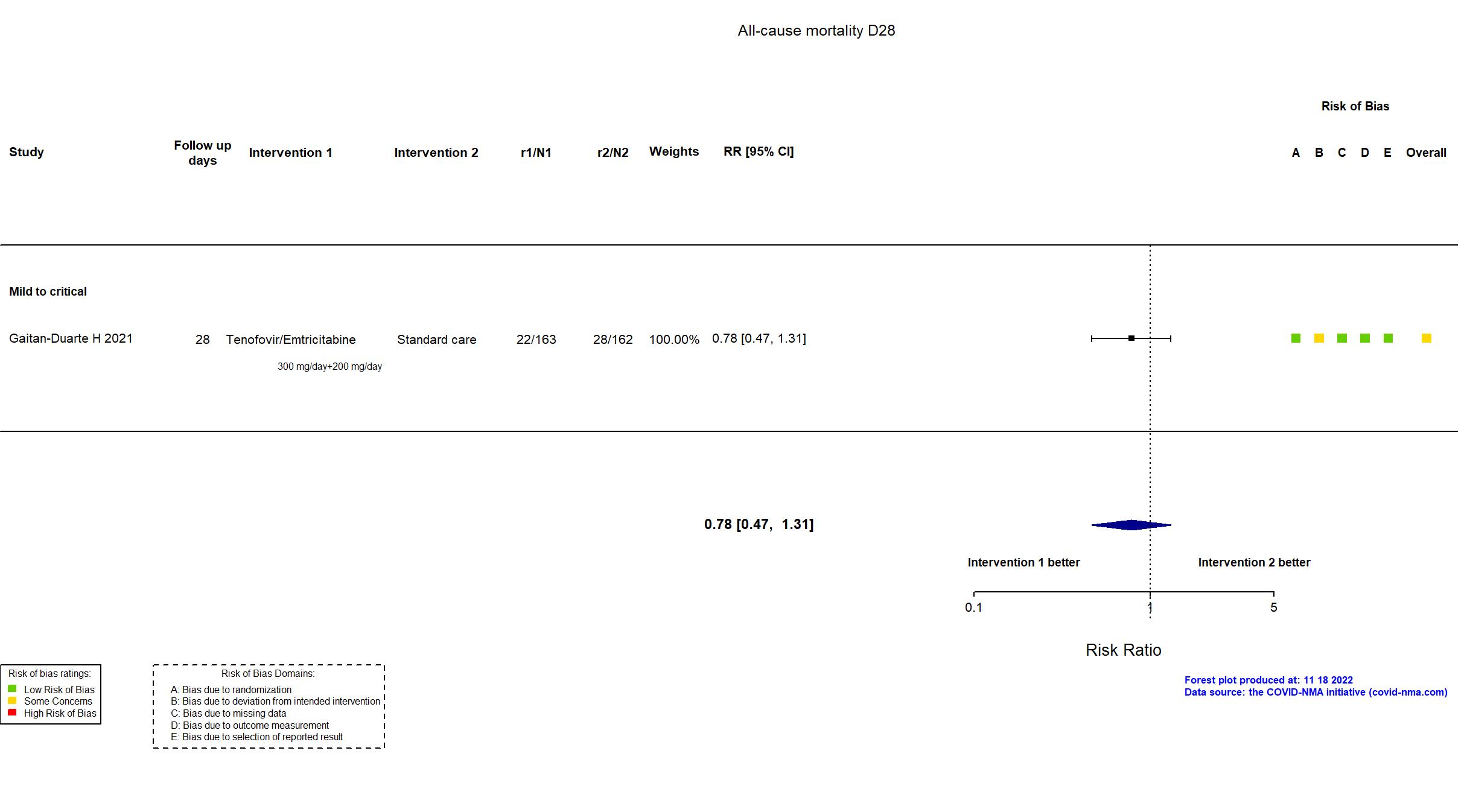



Trial NCT04359095
Publication Gaitan-Duarte H, eClinicalMedicine (2021) (published paper)
Dates: 2020-08-24 to 2021-03-20
Funding: Public/non profit (Colombian Ministry of Science and Technology, Universidad Nacional de Colombia, and Pontificia Universidad Javeriana)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Colombia Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Tenofovir/Emtricitabine Tenofovir: 300 mg orally once daily for 10 days Emtricitabine: 200 mg orally once daily for 10 days CLC+ROSU Colchicine: 0.5 mg orally twice daily for 14 days Rosuvastatin: 40 mg orally once daily for 14 days EMT/TNF+CLC+ROSU Tenofovir: 300 mg orally once daily for 10 days Emtricitabine: 200 mg orally once daily for 10 days Colchicine: 0.5 mg orally twice daily for 14 days Rosuvastatin: 40 mg orally once daily for 14 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Tenofovir/Emtricitabine=163 CLC+ROSU=161 EMT/TNF+CLC+ROSU=163 Standard care=162 | |
| Characteristics of participants N= 649 Mean age : NR 428 males Severity : Mild: n=109 / Moderate: n=418 / Severe: n=57 Critical: n=49 | |
| Primary outcome | |
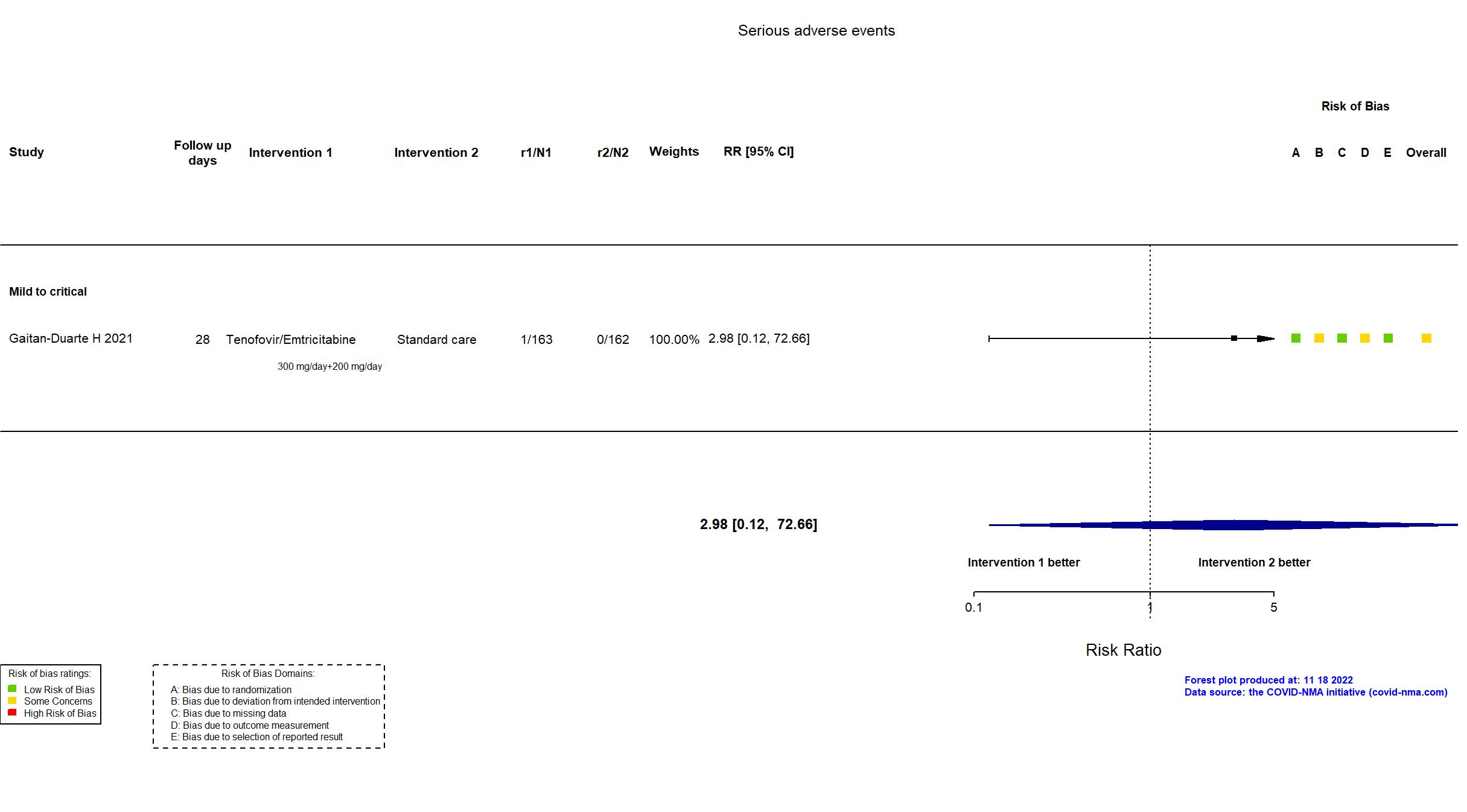
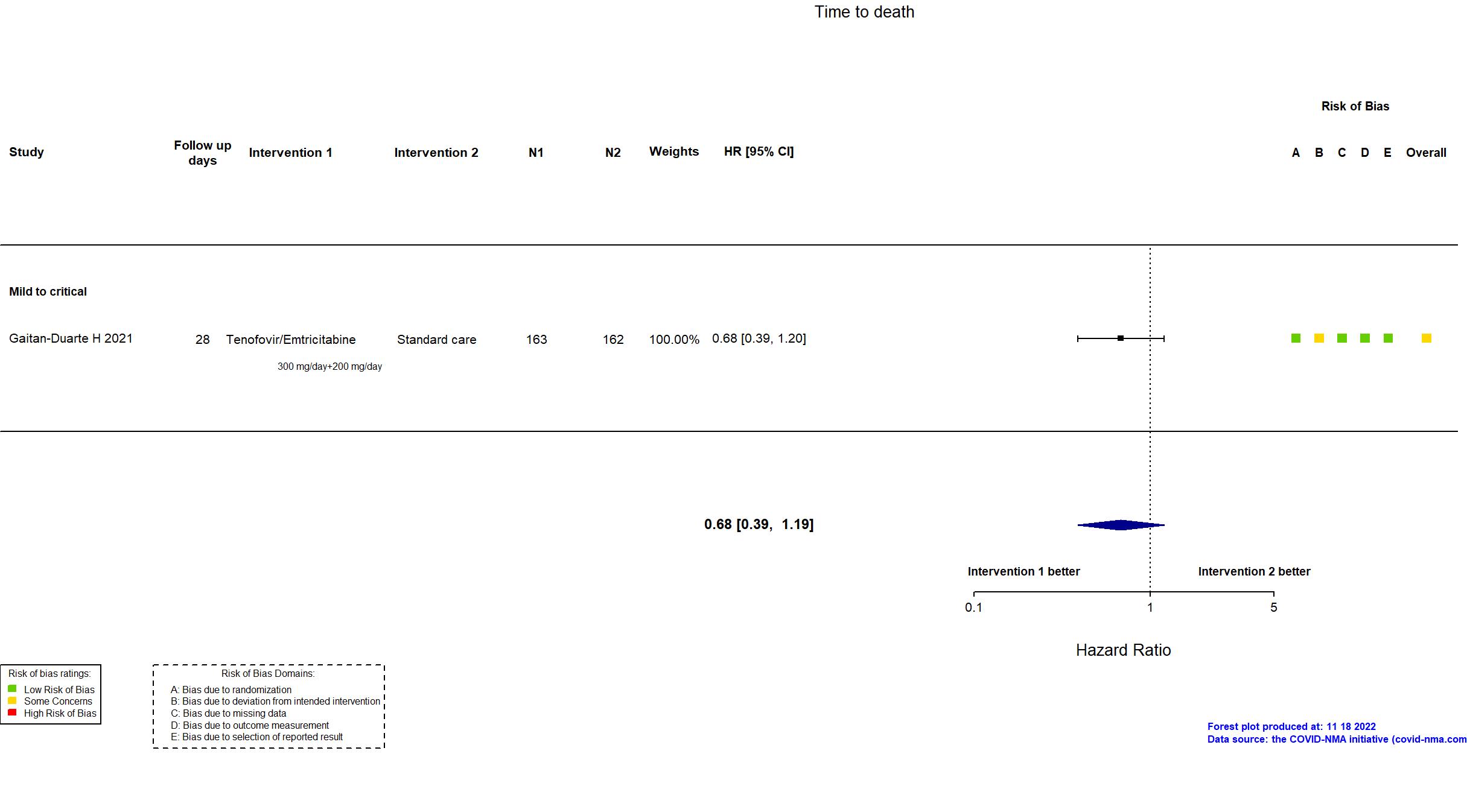
| In the register Mortality [ Time Frame: Post-intervention at day 28 ] Number of Participants with Treatment Related Severe Adverse Events as Assessed by the NCORP Guidance for Collection of Adverse Events Related to COVID-19 Infection [ Time Frame: Post-intervention at day 28 ] Number of participants that develop severe adverse events related to the treatment | |
| In the report All-cause mortality within 28 days after after treatment assignment; Severe adverse events | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the pre-print article, the protocol, statistical analysis plan and the prospective registry were used in data extraction and assessment of risk of bias. There were no major differences between the outcomes planned in the registry and those in the report. The study did not achieve its target sample size.
The study was updated on the March 2nd, 2022 with data extracted from the published report. |