Hydroxychloroquine+Ribavirin vs Standard care (RCT)
Hospitalized patients
FOREST PLOTS -2022-04-12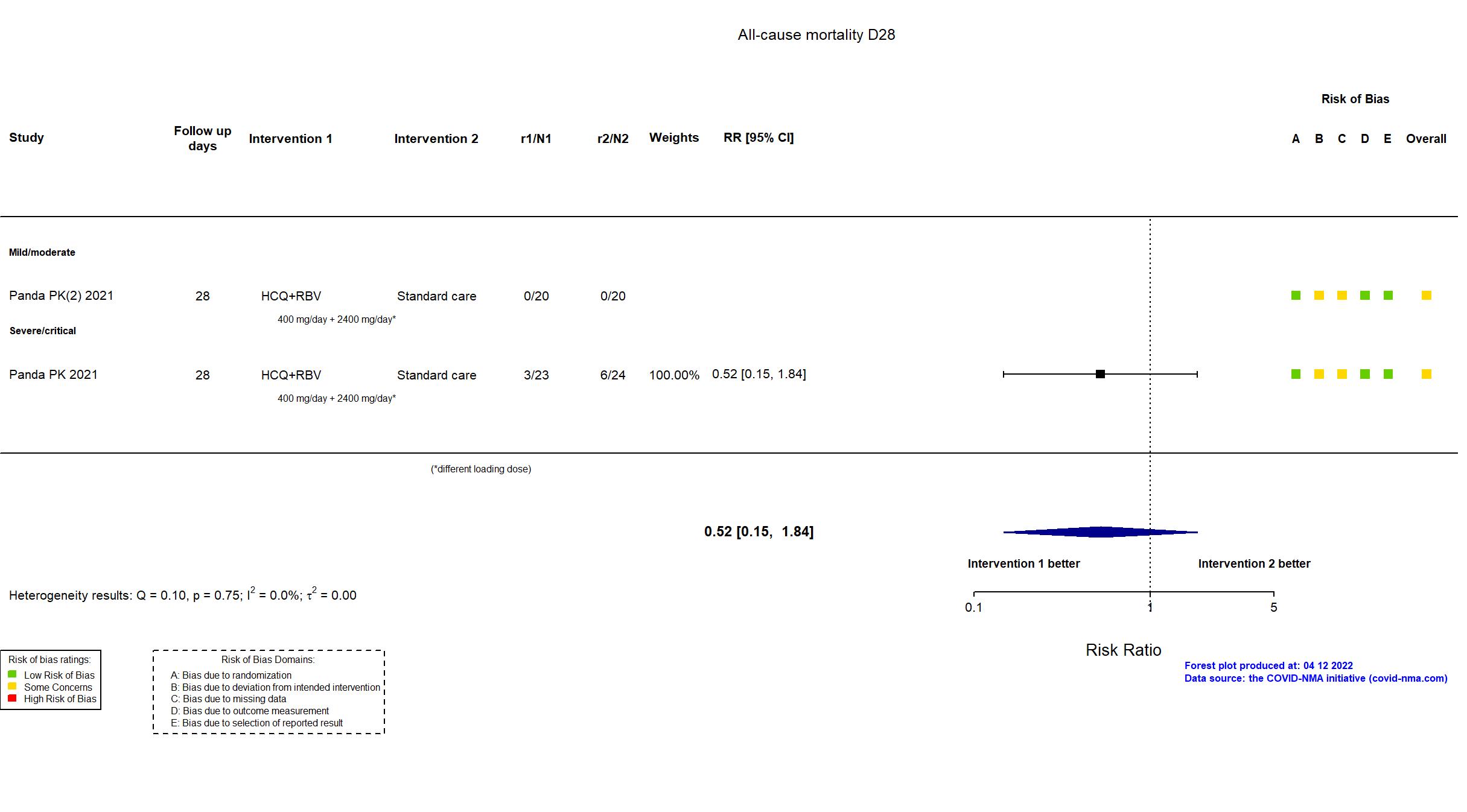

Trial CTRI/2020/06/025575
Publication Panda PK, Clin Pharmacol (2021) (published paper)
Dates: 2020-06-03 to 2020-10-30
Funding: No specific funding (Non-funded)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / India Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
HCQ+RBV Initial dose: HCQ 400mg orally twice a day on day 1 + RBV 2400 mg, Maintenance dose: HCQ 400 mg orally once daily + RBV 1200 mg orally every 12 hours, for 10 days LPV/r+RBV Initial dose: RBV 2400 mg orally as a loading dose.Maintenance dose:LPV/r 400 mg/100 mg orally twice daily + RBV 1200 mg orally every 12 hours, for 10 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : HCQ+RBV=23 LPV/r+RBV=24 Standard care=24 | |
| Characteristics of participants N= 71 Mean age : NR 49 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=* Critical: n=* | |
| Primary outcome | |
| In the register 1. Time to Clinical recovery (TTCR): TTCR is defined as the time (in hours) from initiation of study treatment (active or placebo) until normalisation of fever, respiratory rate, and oxygen saturation, and alleviation of cough, sustained for at least 72 hours. 2. Time to 2019-nCoV RT-PCR negative in upper respiratory tract specimen. | |
| In the report Safety, clinical recovery of symptoms and laboratory recovery of each organ involvement, and time to SARS-CoV-2 RT-PCR negative report of nasopharyngeal/throat swab specimen. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the pre-print article, the published protocol summary, the protocol, and the study registry was used in data extraction and risk of bias assessment. The study did not achieve the target sample size specified in the trial registry. The dosage of medication was not reported in the study, therefore we are unable to determine if the were any changes from the trial registration in the intervention treatments. The drug regimens in the published protocol summary and full protocol differed from those in the registry. The primary outcome indicated in the registry reflects the primary outcome reported in the paper. Only serious adverse events related to the study drug were reported (i.e., non-serious adverse events are not reported). |
Trial CTRI/2020/06/025575
Publication Panda PK(2), Clin Pharmacol (2021) (published paper)
Dates: 2020-06-03 to 2020-10-30
Funding: No specific funding (Non-funded)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / India Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
HCQ+RBV Initial dose: RBV 2400 mg orally as a loading dose. Maintenance dose: HCQ 400mg orally twice daily on day 1 followed by 400 mg orally once daily + RBV 1200 g orally every 12 hours, for 10 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : HCQ+RBV=20 Standard care=20 | |
| Characteristics of participants N= 40 Mean age : NR 26 males Severity : Mild: n=28 / Moderate: n=5 / Severe: n=0 Critical: n=0 | |
| Primary outcome | |
| In the register 1. Time to Clinical recovery (TTCR): TTCR is defined as the time (in hours) from initiation of study treatment (active or placebo) until normalisation of fever, respiratory rate, and oxygen saturation, and alleviation of cough, sustained for at least 72 hours. 2. Time to 2019-nCoV RT-PCR negative in upper respiratory tract specimen. | |
| In the report Safety, clinical recovery of symptoms and laboratory recovery of each organ involvement, and time to SARS-CoV-2 RT-PCR negative report of nasopharyngeal/throat swab specimen. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the pre-print article, the study registry was used in data extraction and risk of bias assessment. The study did not achieve the target sample size specified in the trial registry. The dosage of medication was not reported in the study, therefore we are unable to determine if the were any changes from the trial registration in the intervention treatments. The primary outcome indicated in the registry reflects the primary outcome reported in the paper. Only serious adverse events were reported (i.e., non-serious adverse events are not reported). |