Tofacitinib vs Standard care/Placebo (RCT)
Hospitalized patients
FOREST PLOTS -2022-03-04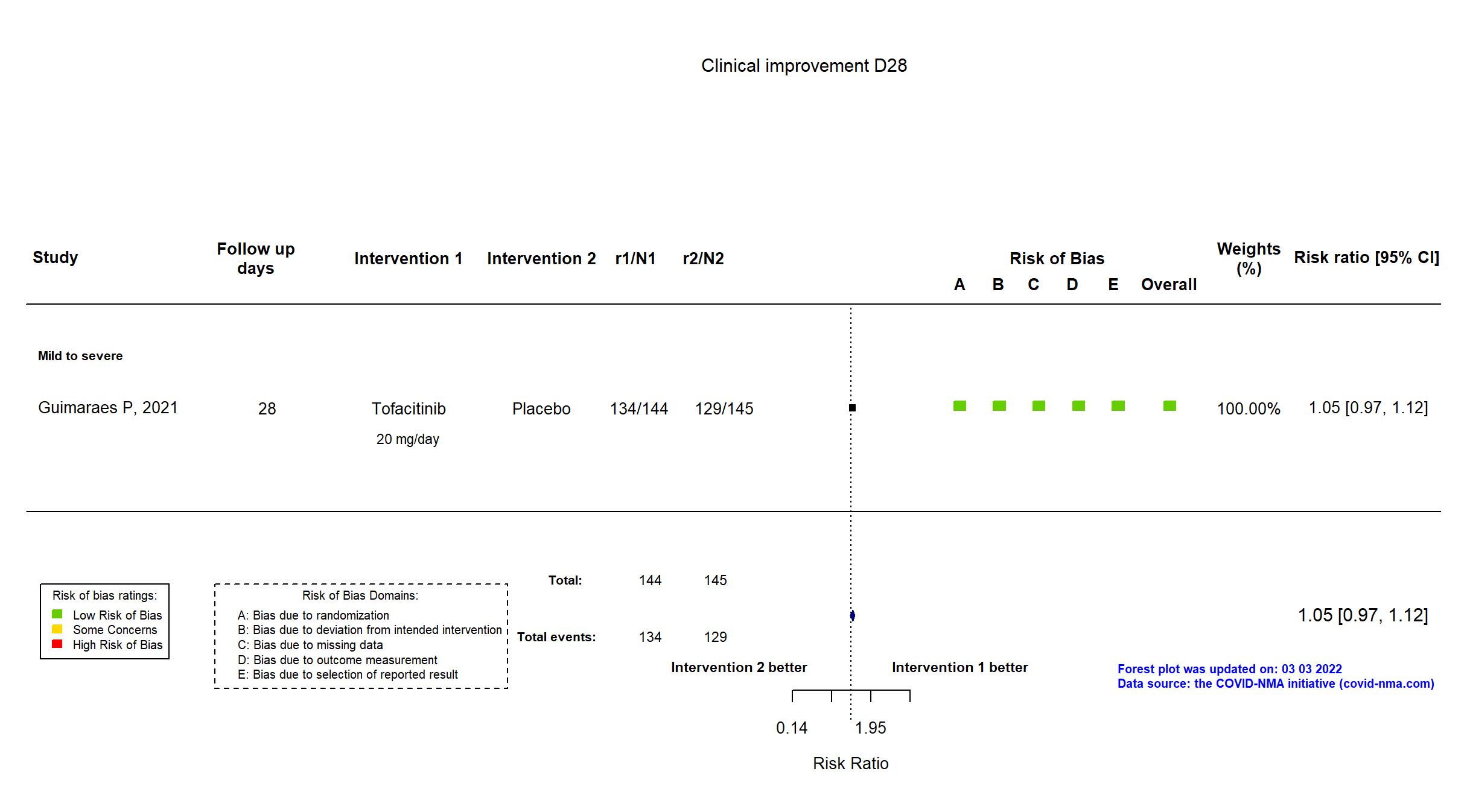
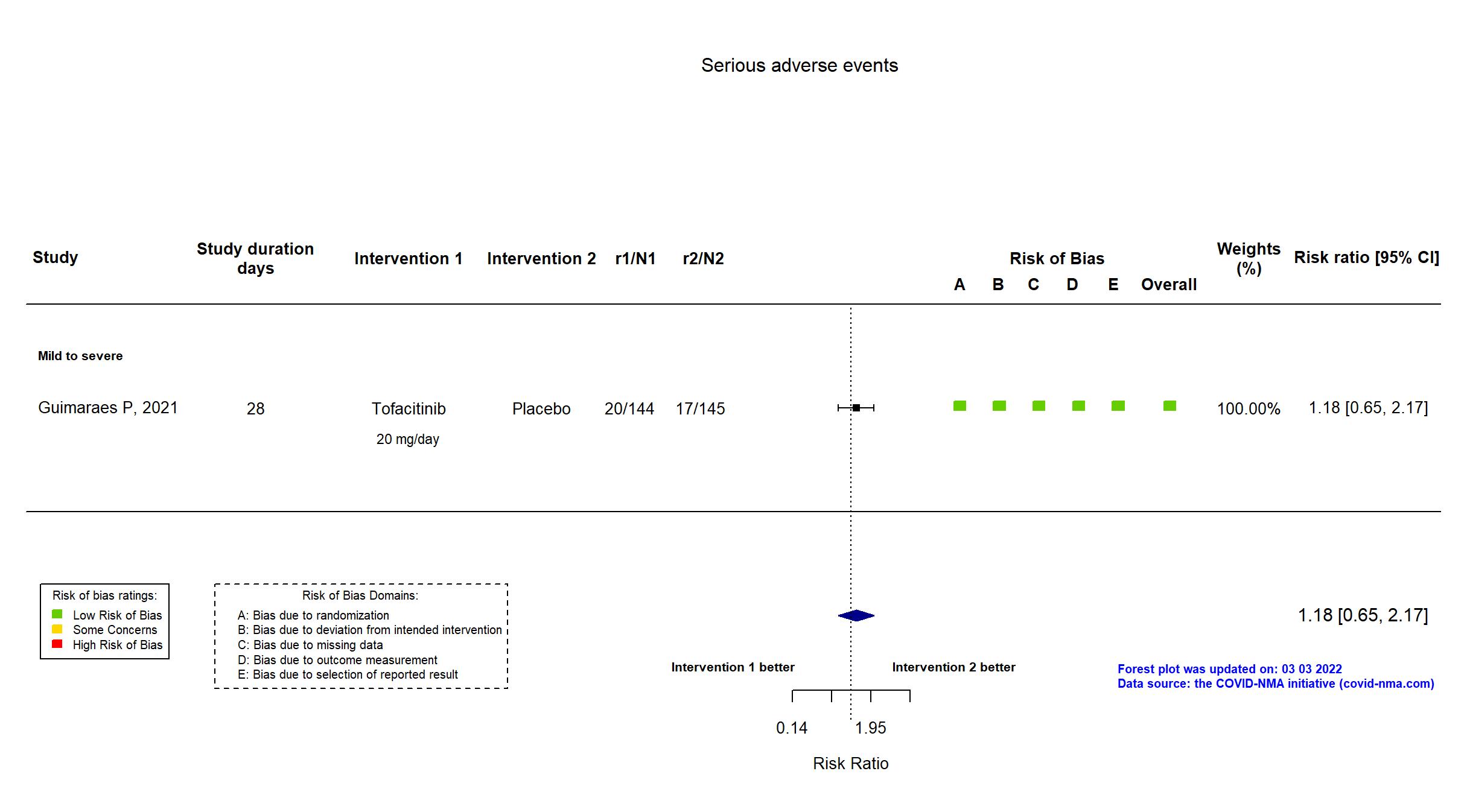
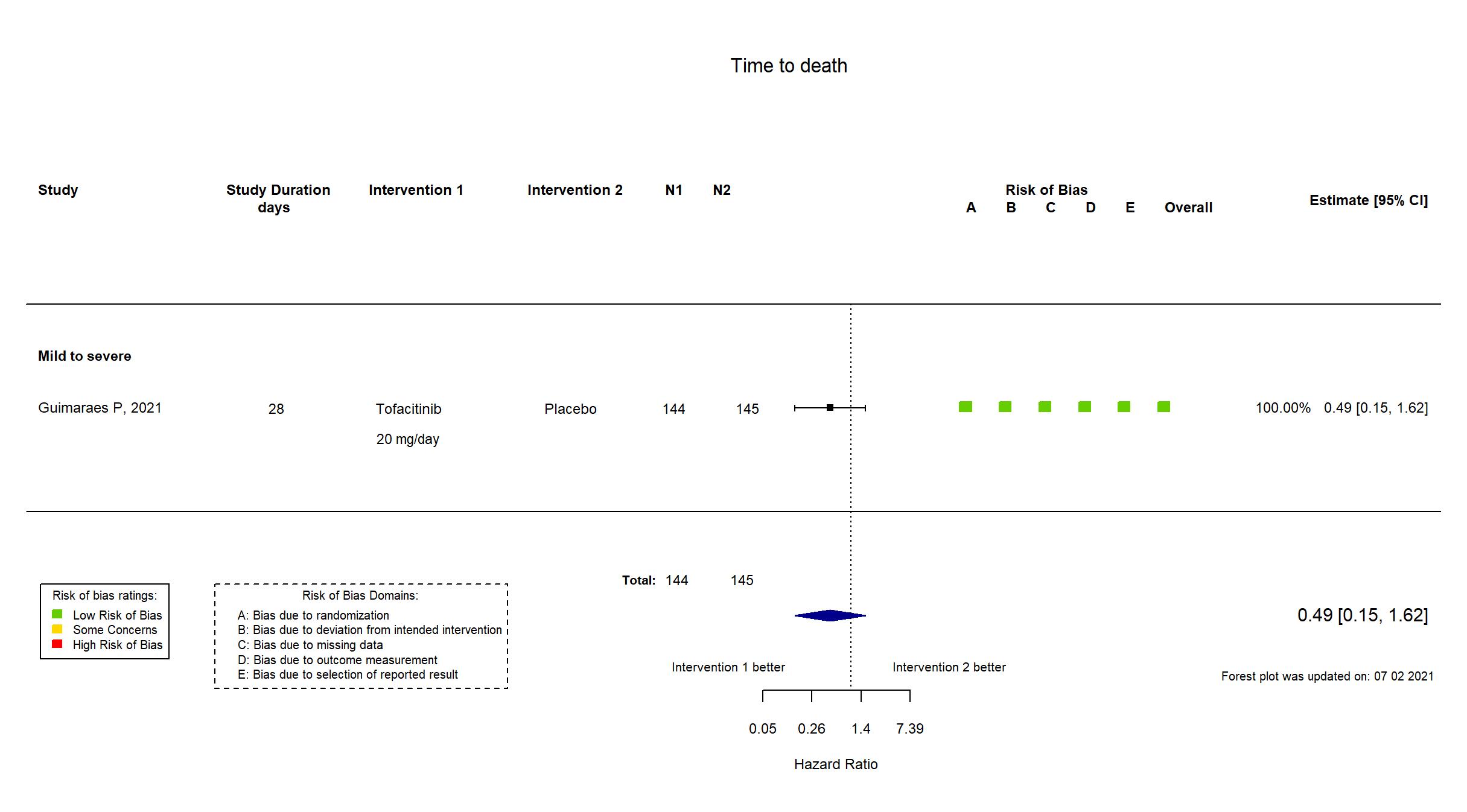
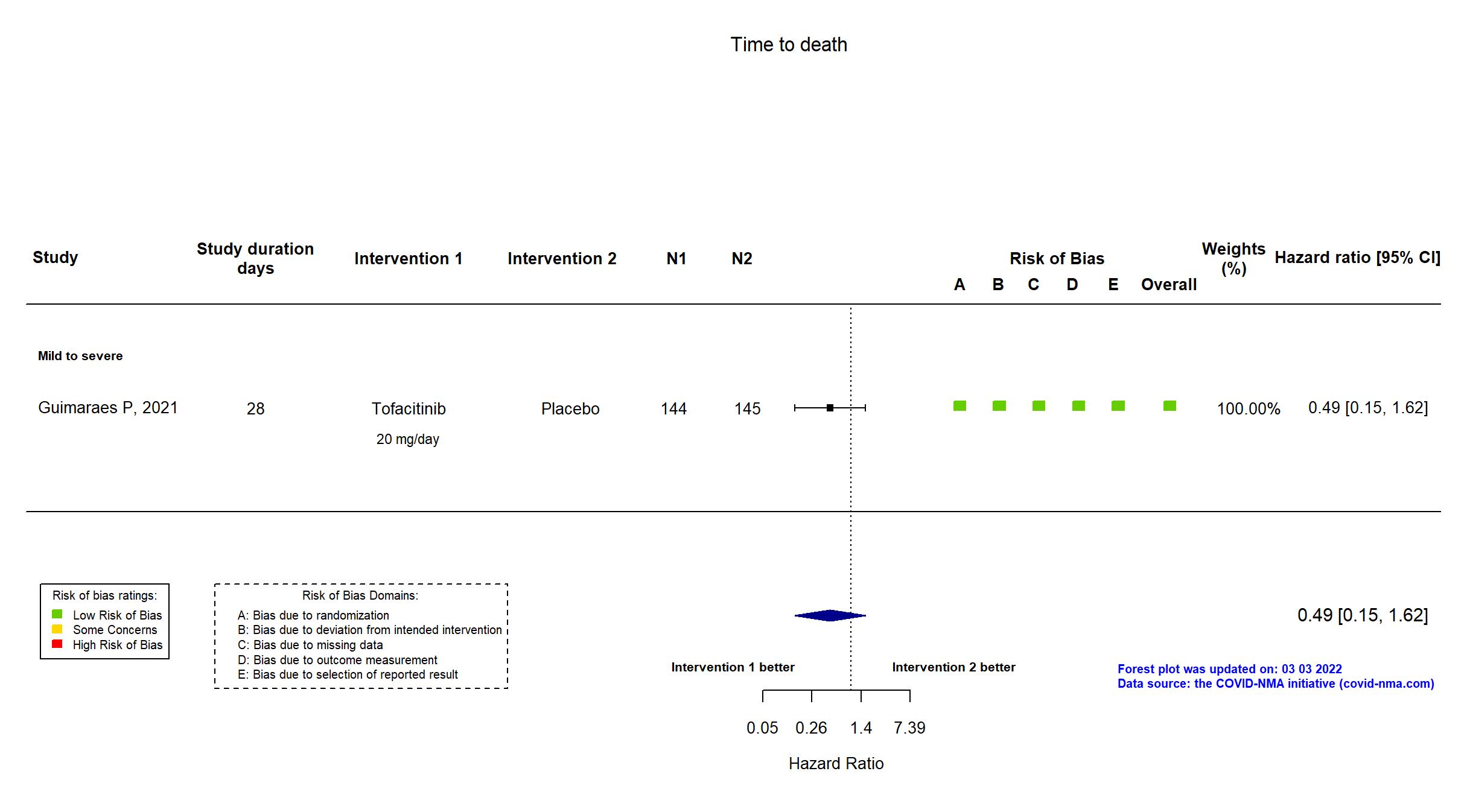







Trial NCT04469114
Publication STOP-COVID - Guimaraes P, N Engl J Med (2021) (published paper)
Dates: 2020-09-16 to 2020-12-13
Funding: Private (Pfizer)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / Brazil Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Tofacitinib 10 mg orally twice a day for up to 14 days or until hospital discharge, whichever was earlier |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Tofacitinib =144 Placebo=145 | |
| Characteristics of participants N= 289 Mean age : NR 188 males Severity : Mild: n=71 / Moderate: n=181 / Severe: n=37 Critical: n=0 | |
| Primary outcome | |
| In the register Death or respiratory failure until Day 28 [ Time Frame: 28 days ] | |
| In the report Death or respiratory failure during the 28 days of follow-up | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: N |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Low |
| General comment | In addition to the published article with supplementary appendices, the protocol with statistical analysis plan and prospective study registry were used in data extraction and risk of bias assessment. The study achieved the target sample size specified in the trial registry. There no substantive differences between the published article and the registry or protocol in population, procedures, outcomes or intervention and control treatments. |
Trial *
Publication Murugesan H , J Assoc Physicians India (2021) (published paper)
Dates: 2020-10-01 to 2020-12-30
Funding: Private (Pfizer)
Conflict of interest: *
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / India Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Tofacitinib 10 mg orally twice a day for 14 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Tofacitinib =50 Standard care=50 | |
| Characteristics of participants N= 100 Mean age : NR 74 males Severity : Mild: n=0 / Moderate: n=* / Severe: n=* Critical: n=0 | |
| Primary outcome | |
| In the register NR | |
| In the report Proportion of patients not requiring any form of mechanical ventilation or high flow oxygen or ECMO at day 7 or mortality. | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | Only the published article was used in data extraction and assessment of risk of bias. No registry, protocol or statistical analysis plan was available, so it was not possible to determine whether the population, procedures, intervention and outcomes were predefined. This was a pilot study with a convenience sample of 100 patients. |