Sotrovimab vs Placebo (RCT)
Mild outpatients
FOREST PLOTS -2022-11-17
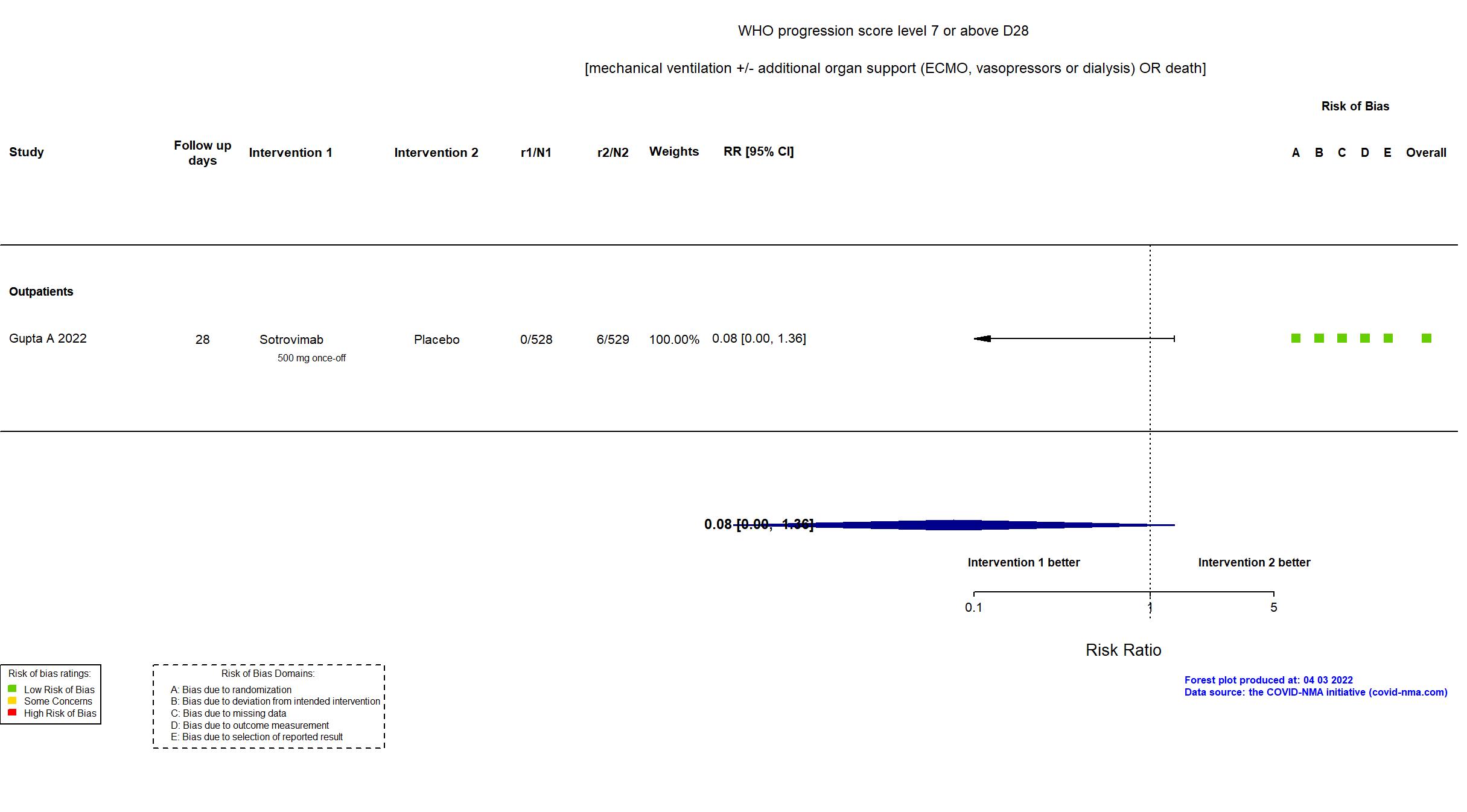
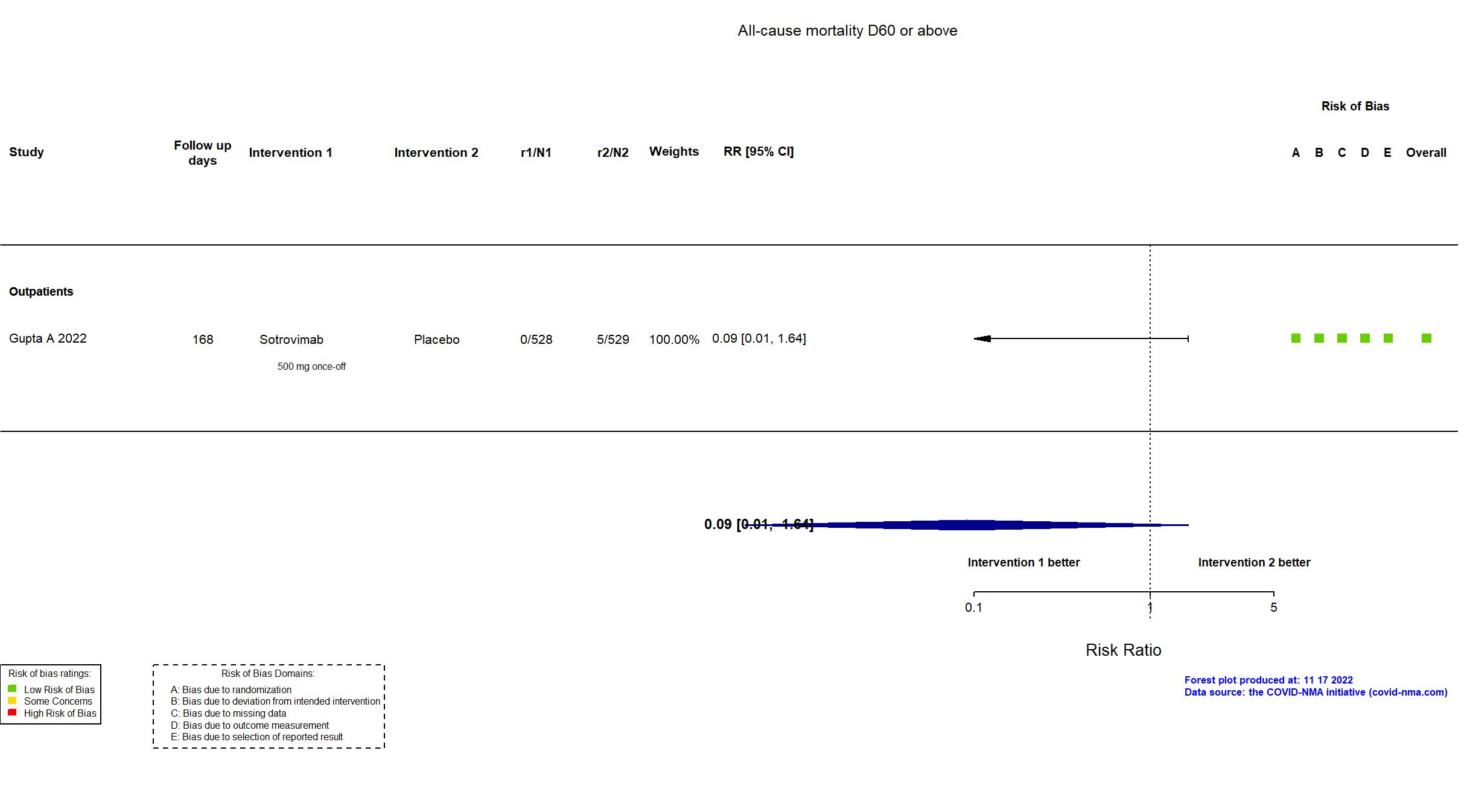
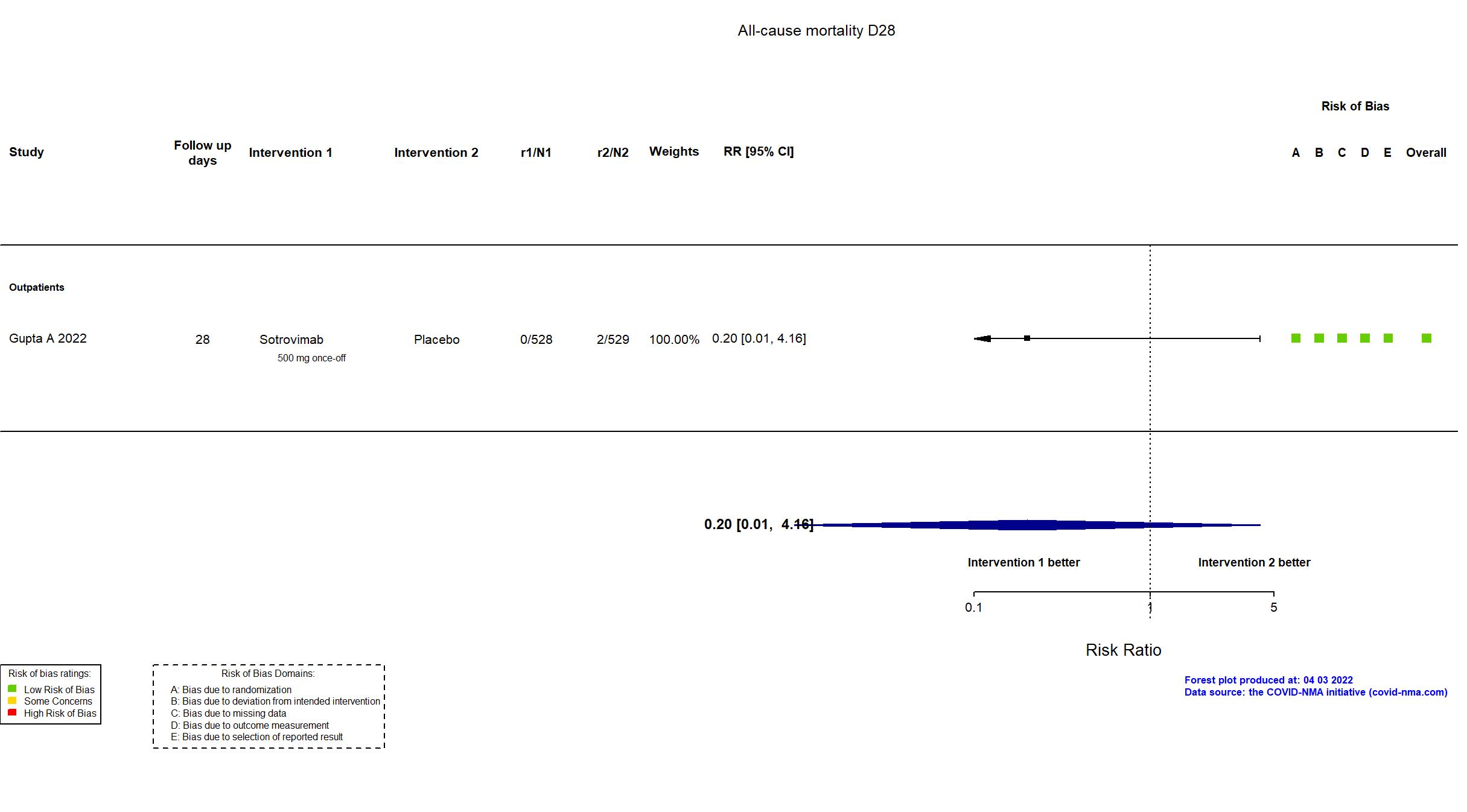
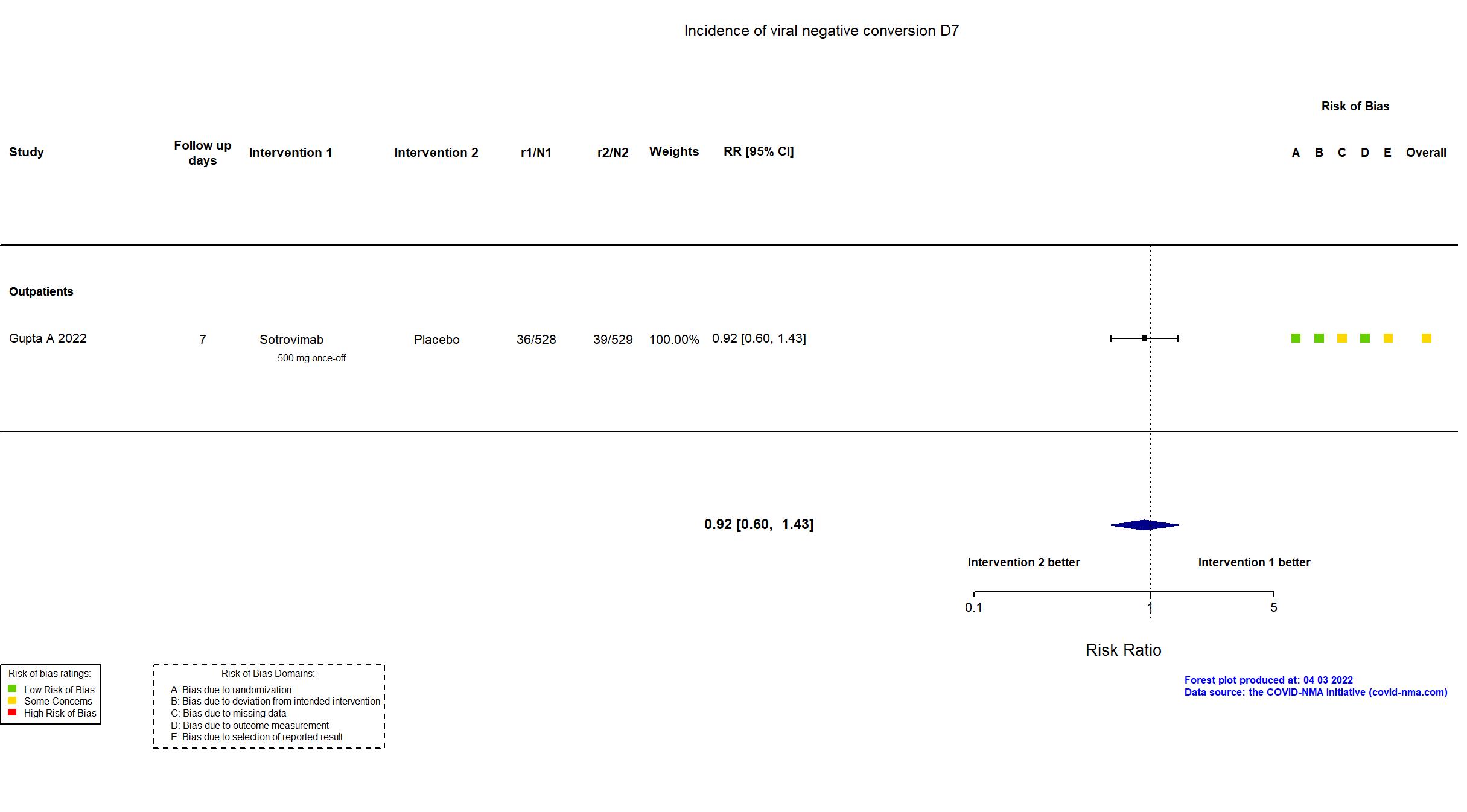
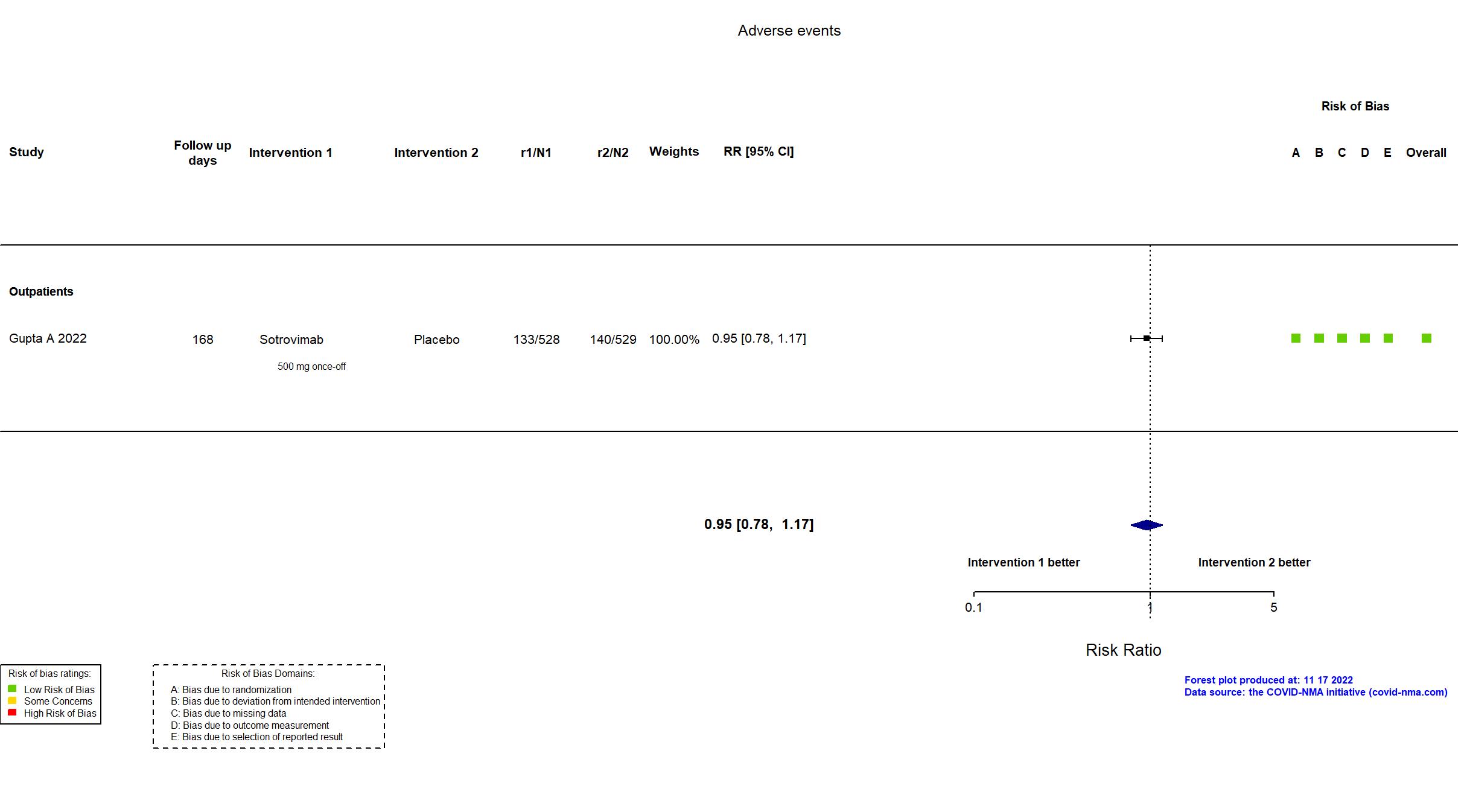
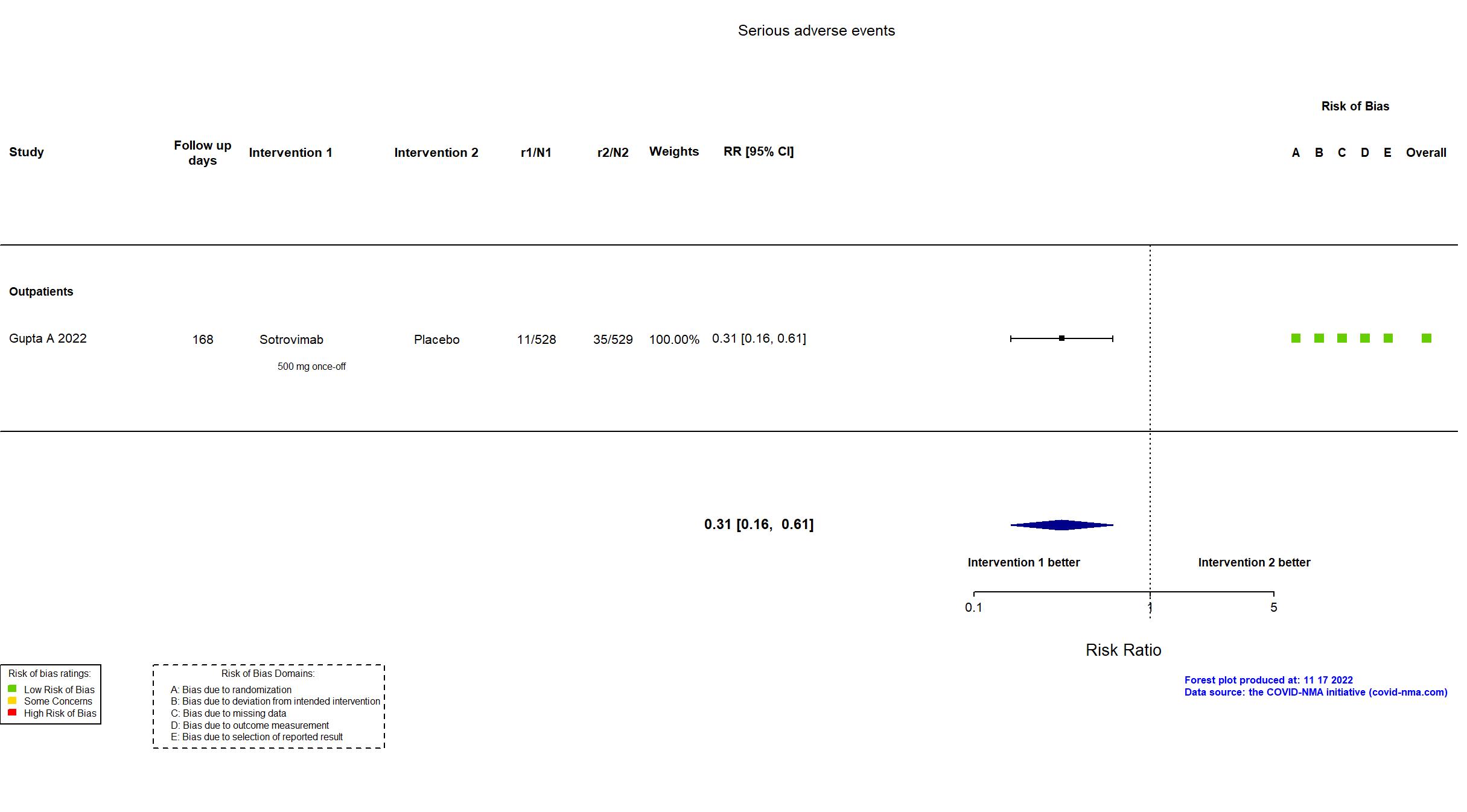







Trial NCT04545060
Publication COMET-ICE - Gupta A, JAMA (2022) (published paper)
Dates: 2020-08-27 to 2021-03-11
Funding: Private (Vir Biotechnology, Inc. and GlaxoSmithKline.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / USA, Canada, Brazil, Spain and Peru Follow-up duration (days): 168 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Sotrovimab 500 mg 1-hour IV infusion on day 1 |
|
| Control
Placebo Equal volume saline placebo. | |
| Participants | |
| Randomized participants : Sotrovimab=528 Placebo=529 | |
| Characteristics of participants N= 1057 Mean age : NR 485 males Severity : Mild: n= 1057/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register Proportion of participants who have progression of COVID-19 through Day 29 [ Time Frame: Up to Day 29 ] | |
| In the report The proportion of patients with all-cause hospitalization for more than 24 hours or death through day 29. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: N |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published/preprint articles of the final results and the published/preprint articles of the interim results, the study registry, protocol, statistical analysis plan and the supplementary material were used in data extraction and risk of bias assessment. The study did not achieved the target sample size specified in the trial registry. "As a result of profound efficacy, an independent data monitoring committee recommended that enrollment in the study be stopped on March 10, 2021, at which time 1057 patients had been randomized". There is no change from the trial registration in the intervention and control treatments although the dose is not specified in the registry. The primary outcome indicated in registry reflects the primary outcome reported in the paper but the registry does not define progression (hospitalization longer than 24 hours or death in the report). The New England Journal of Medicine 2021 article reports results of a pre-planned interim analysis triggered when approximately 41% of the required number of study patients reached day 29 of follow-up. "Additional efficacy, safety, and laboratory data, as well as initial immunogenicity data, will be reported subsequently." This is in the JAMA 2022 article. The outcomes "hospitalization or death" and "WHO score 7 and above" were extracted through day 28.
The study was updated on November 15th, 2021 with data from a preprint article with all final results. The study was updated on January 5th, 2022 with data from a published abstract. The study was updated on March 28th with data from the published report with final results. The study was updated on November 18th, 2022 with data extracted from the registry with longer follow up (up to 24 weeks). |