Sotrovimab vs Placebo (RCT)
Hospitalized patients
FOREST PLOTS -2022-01-04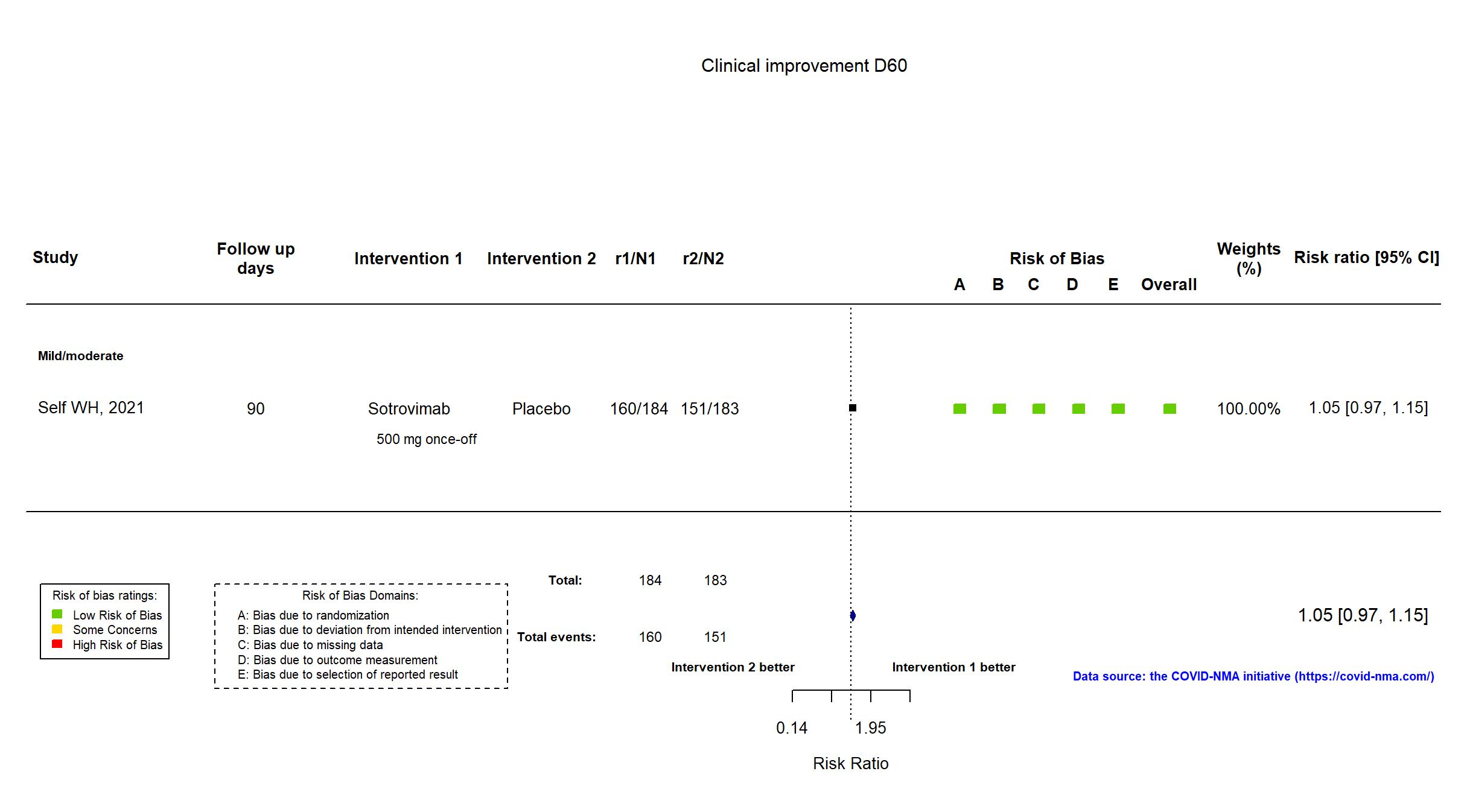
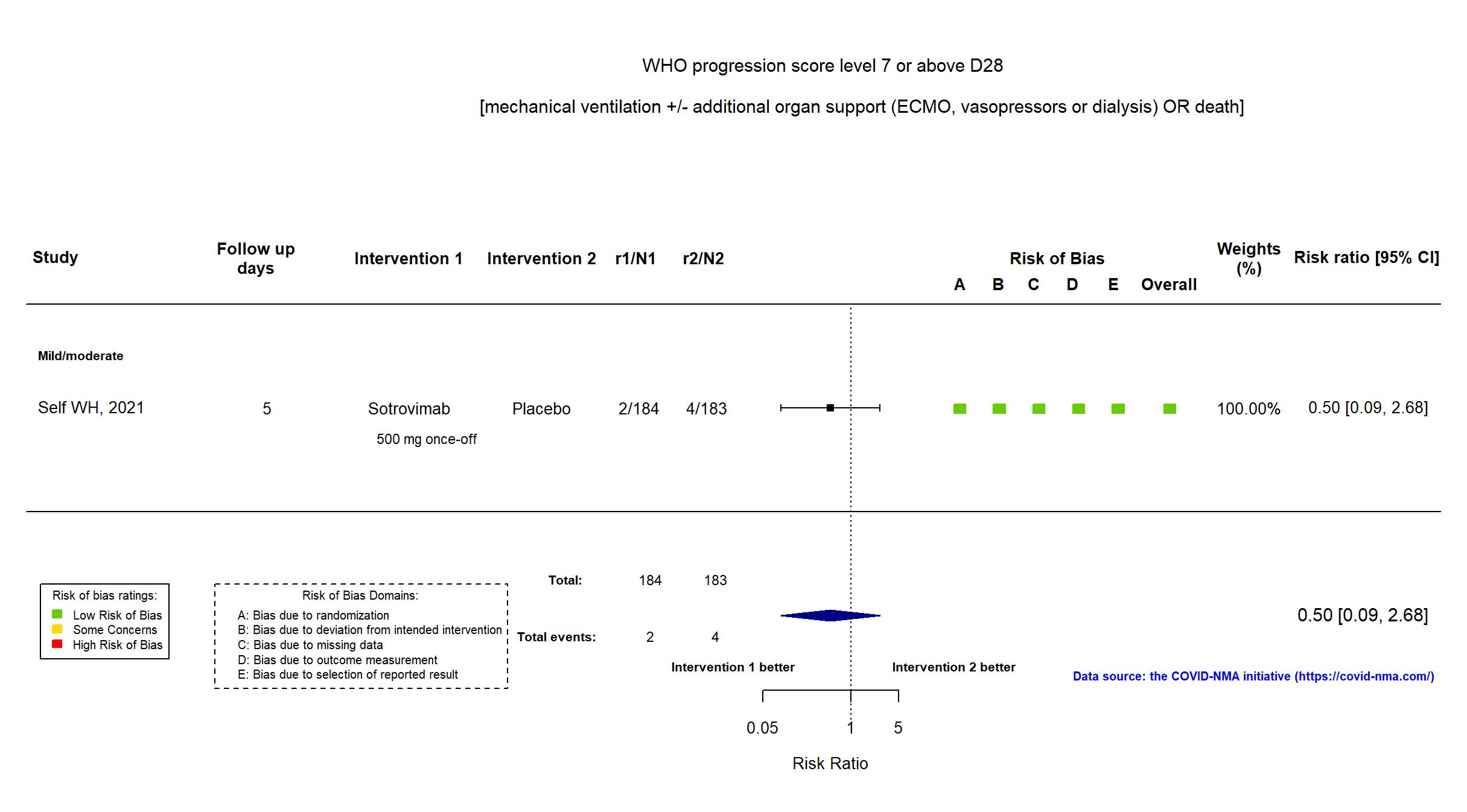
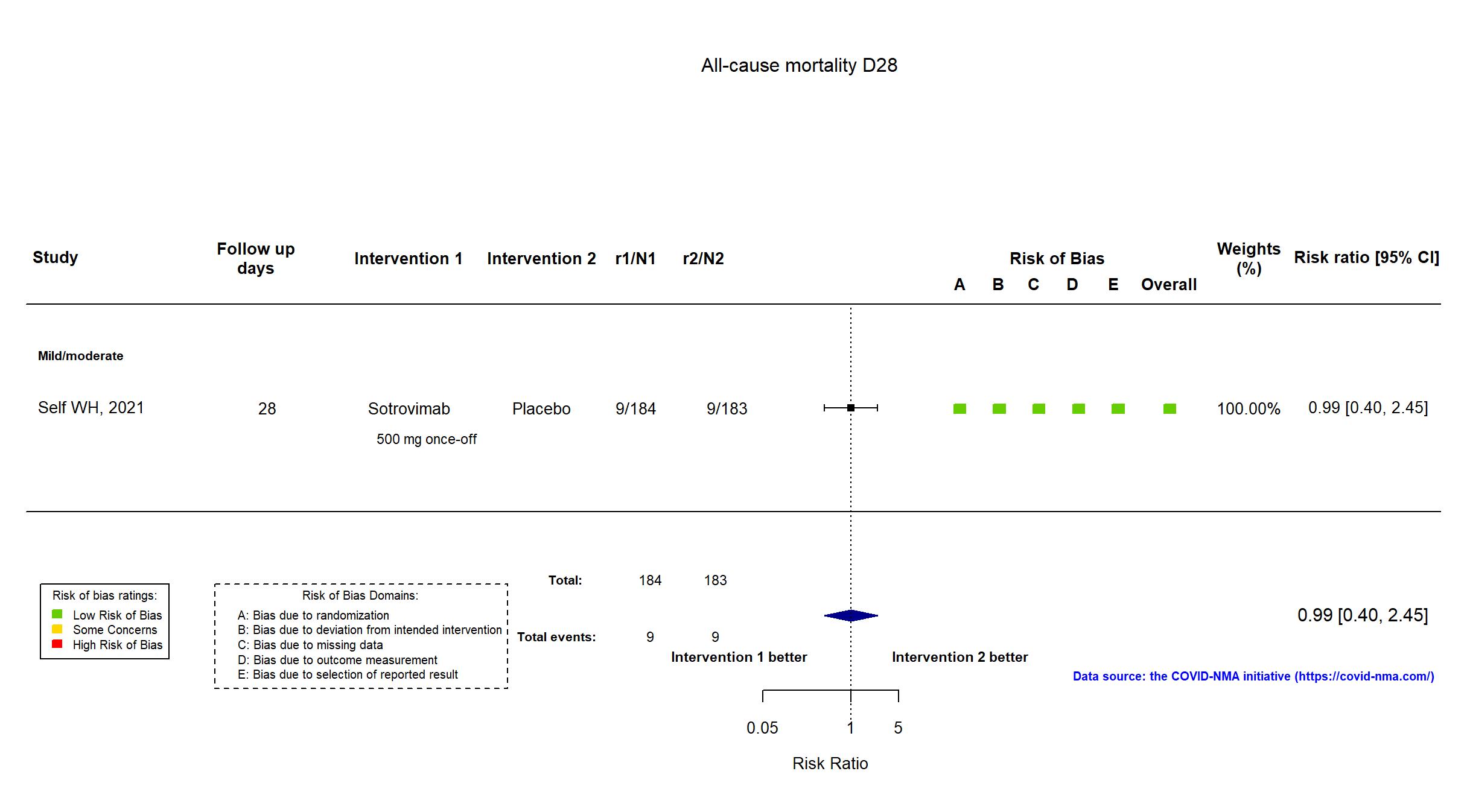
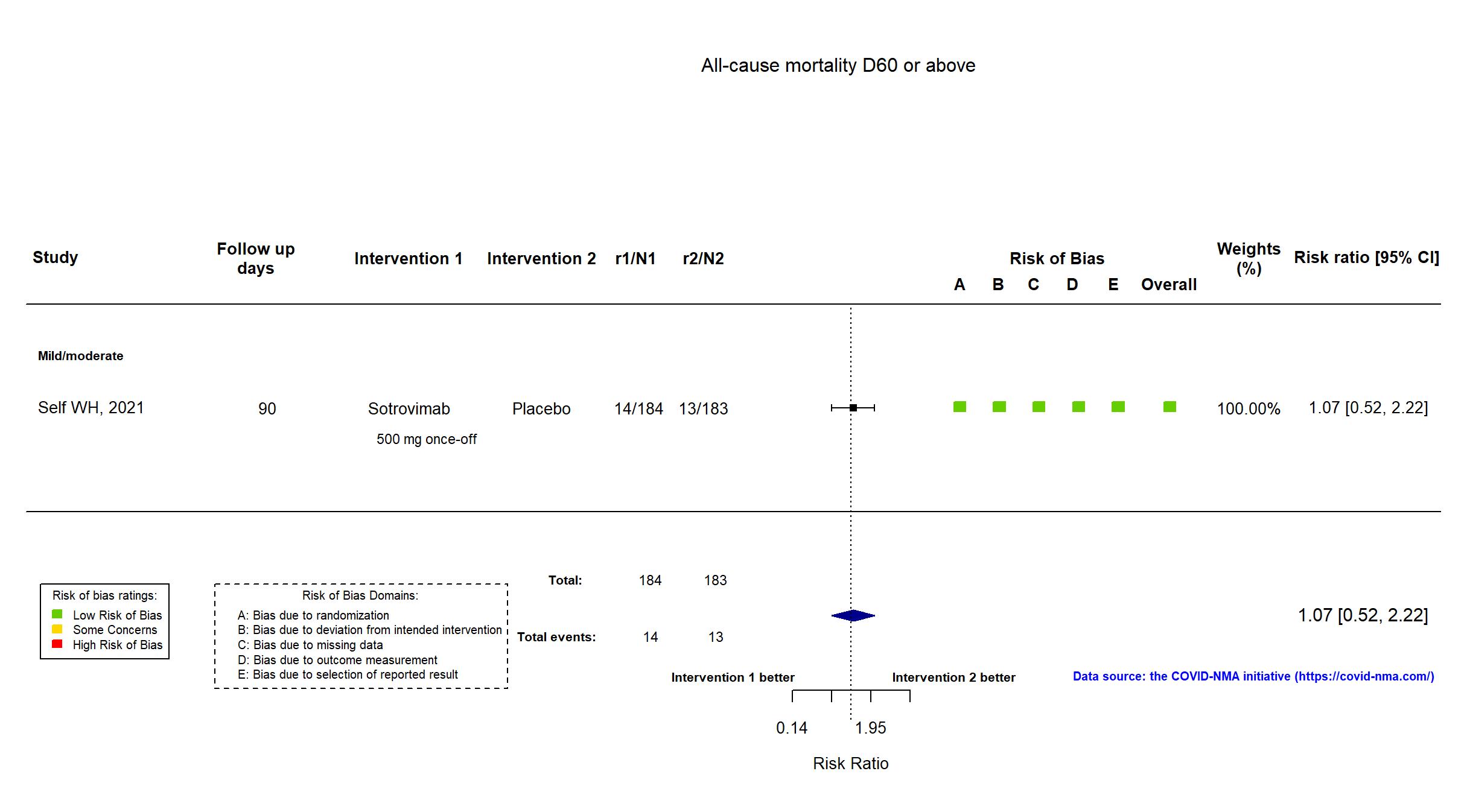
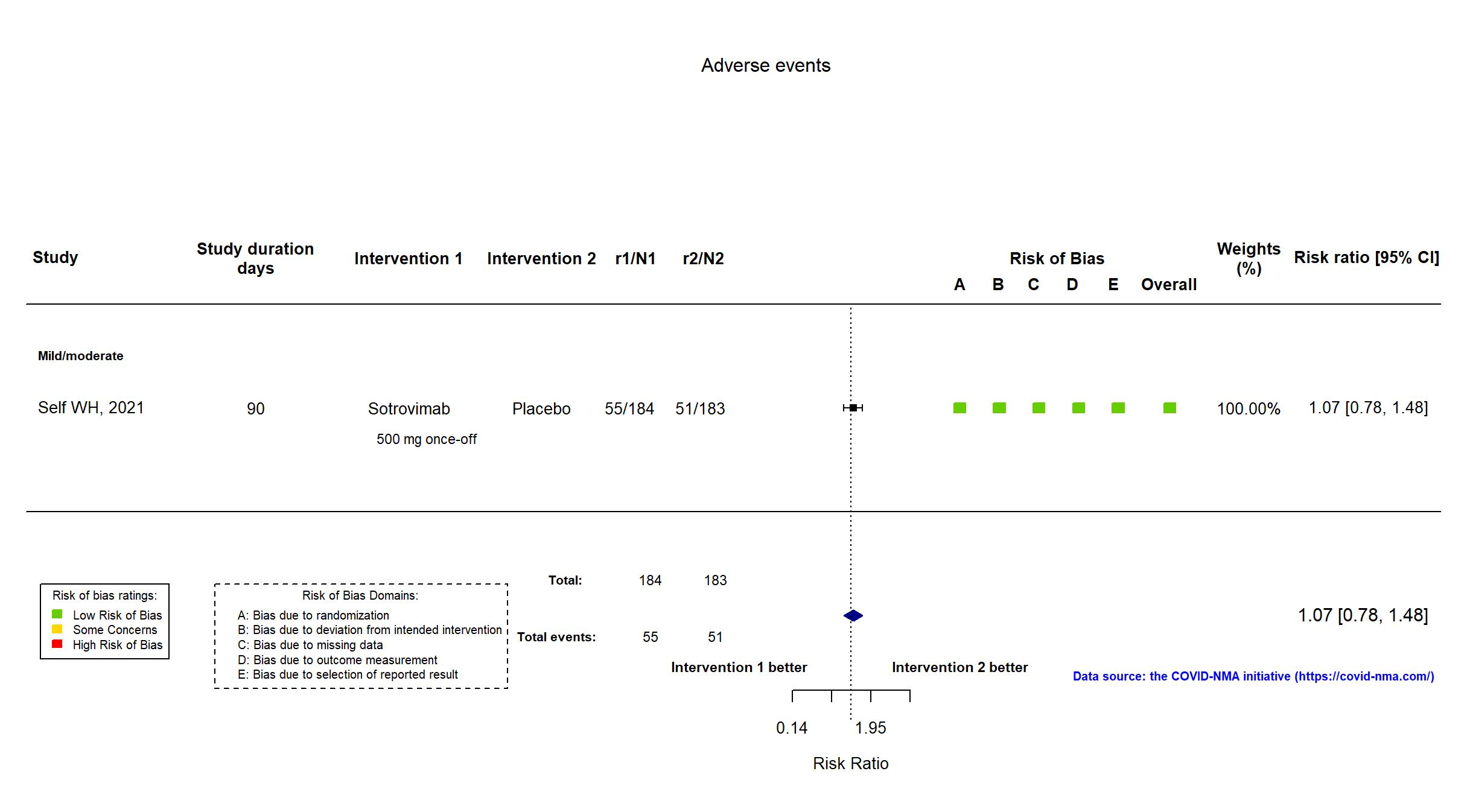
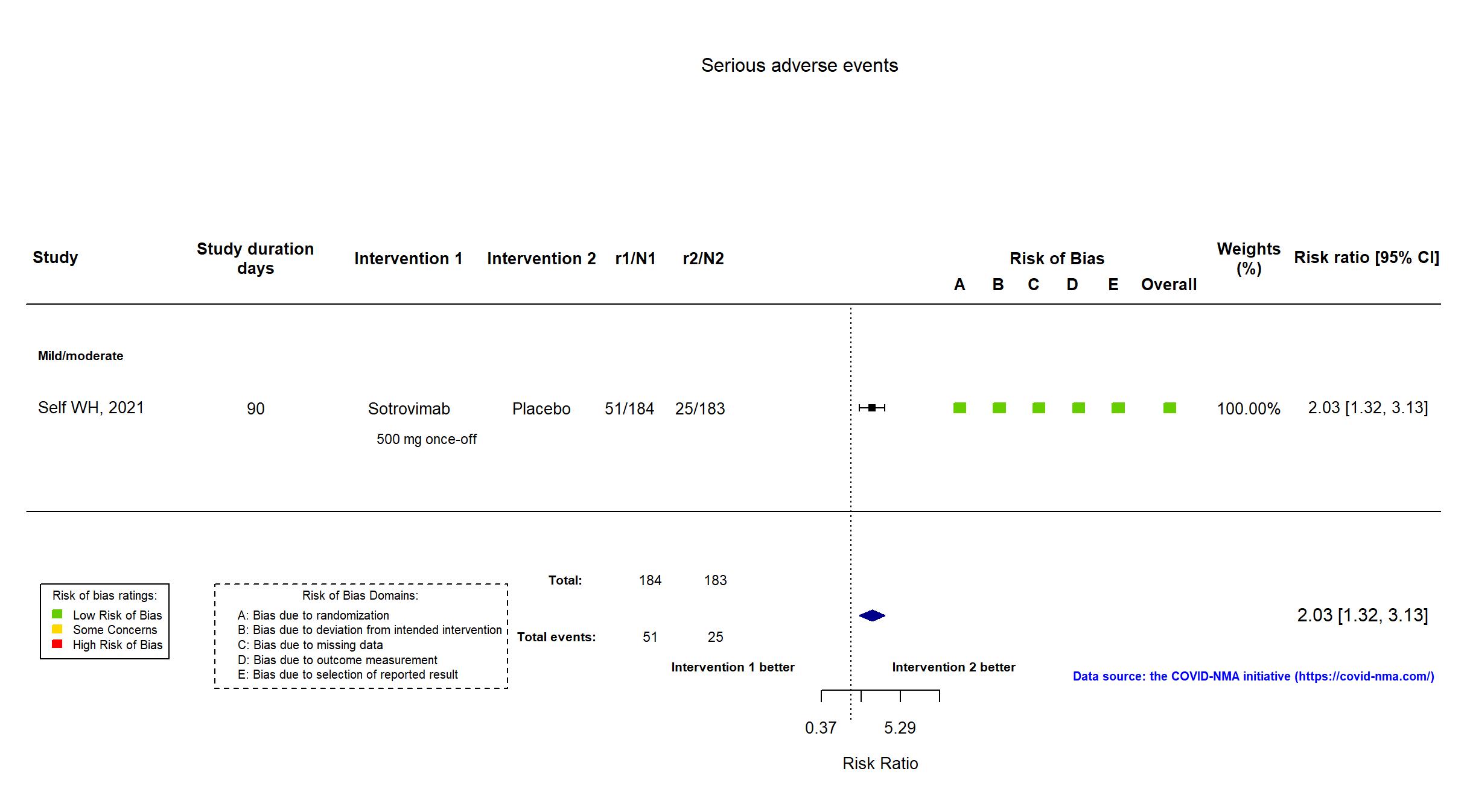
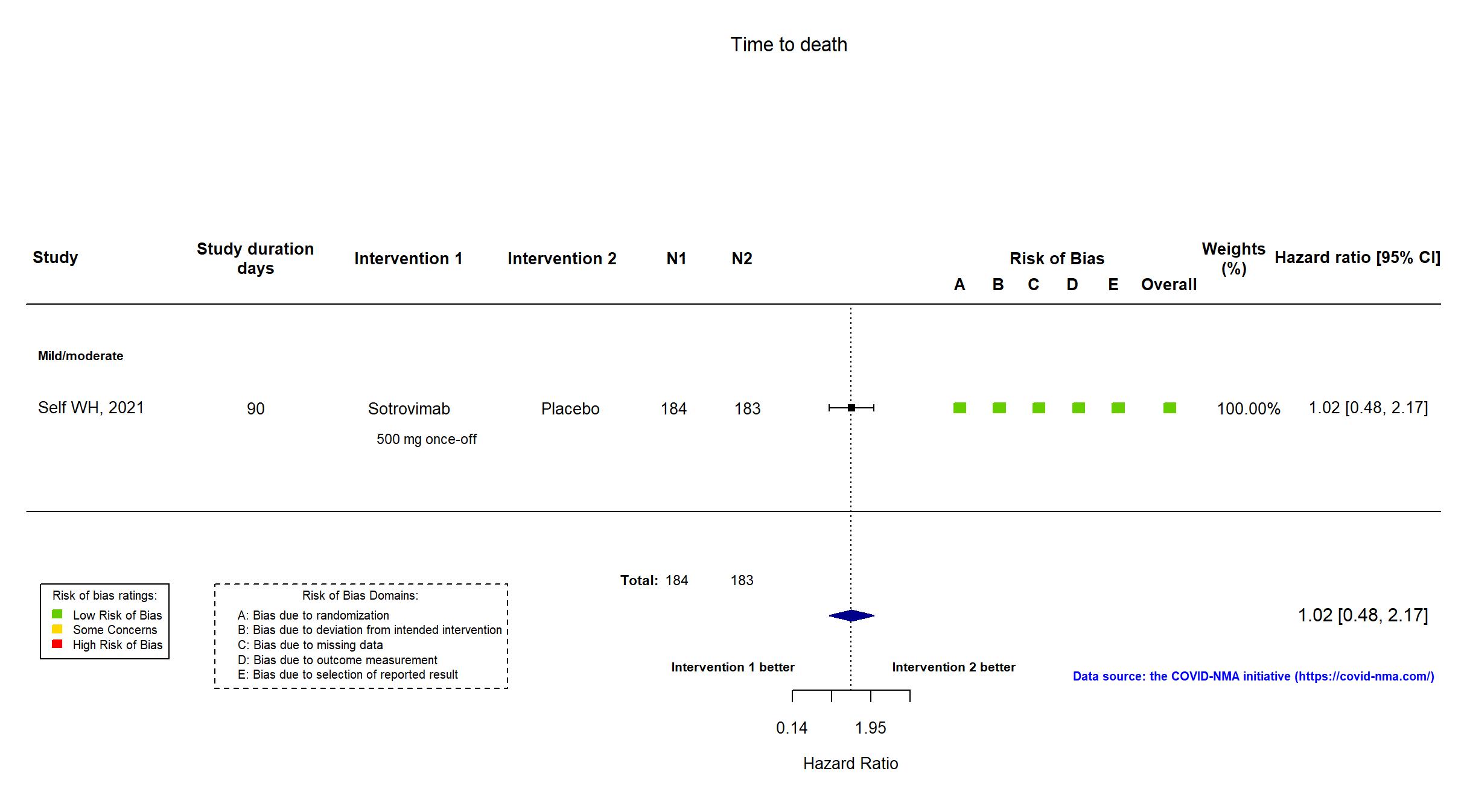







Trial NCT04501978
Publication ACTIV-3 - Self WH, Lancet Infect Dis (2021) (published paper)
Dates: 2020-12-16 to 2021-03-01
Funding: Mixed (Medications provided by Vir/GlaxoSmithKline; Brii Biosciences; Gilead Sciences; US Operation Warp Speed programme, NIAID; Leidos Biomedical Research for the INSIGHT Network; NHLBI; the Research Triangle Institute for the Prevention; Early Treatment of Acute Lung Injury Network; Cardiothoracic Surgical Trials Network; the Office of Research & Development; US Department of Veterans Affairs; governments of Denmark, United Kingdom; NIH.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / Denmark, Poland, Switzerland, USA Follow-up duration (days): 90 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Sotrovimab 500 mg intravenously once-off over 60 minutes BRII-196/BRII-198 1000 BRII-196 mg + 1000 mg BRII-198 intravenously once-off over 60 minutes |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Sotrovimab=184 Placebo=183 BRII-196/BRII-198 =179 | |
| Characteristics of participants N= 546 Mean age : NR 308 males Severity : Mild: n=176 / Moderate: n=360 / Severe: n=0 Critical: n=0 | |
| Primary outcome | |
| In the register Time from randomization to sustained recovery [ Time Frame: Up to Day 90 ] Sustained recovery defined as being discharged from the index hospitalization, followed by being alive and home for 14 consecutive days prior to Day 90. | |
| In the report Time to sustained clinical recovery up to day 90, defined as time between randomisation and return to home (with home defined as the patient's residence before COVID-19 or a location that provided similar or less intensive medical care) for 14 consecutive days. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Low |
| General comment | In addition to the published article, the trial registry, protocol, statistical analysis plan and supplementary appendices were used in data extraction and assessment of risk of bias. The primary outcome in the article reflected that in the registry. The study (n = 546) achieved the target sample size for the early futility assessment (n = 450), but recruitment was terminated as a result of the interim analysis so that the study did not achieve the target sample size for efficacy assessment (n = 1000). |