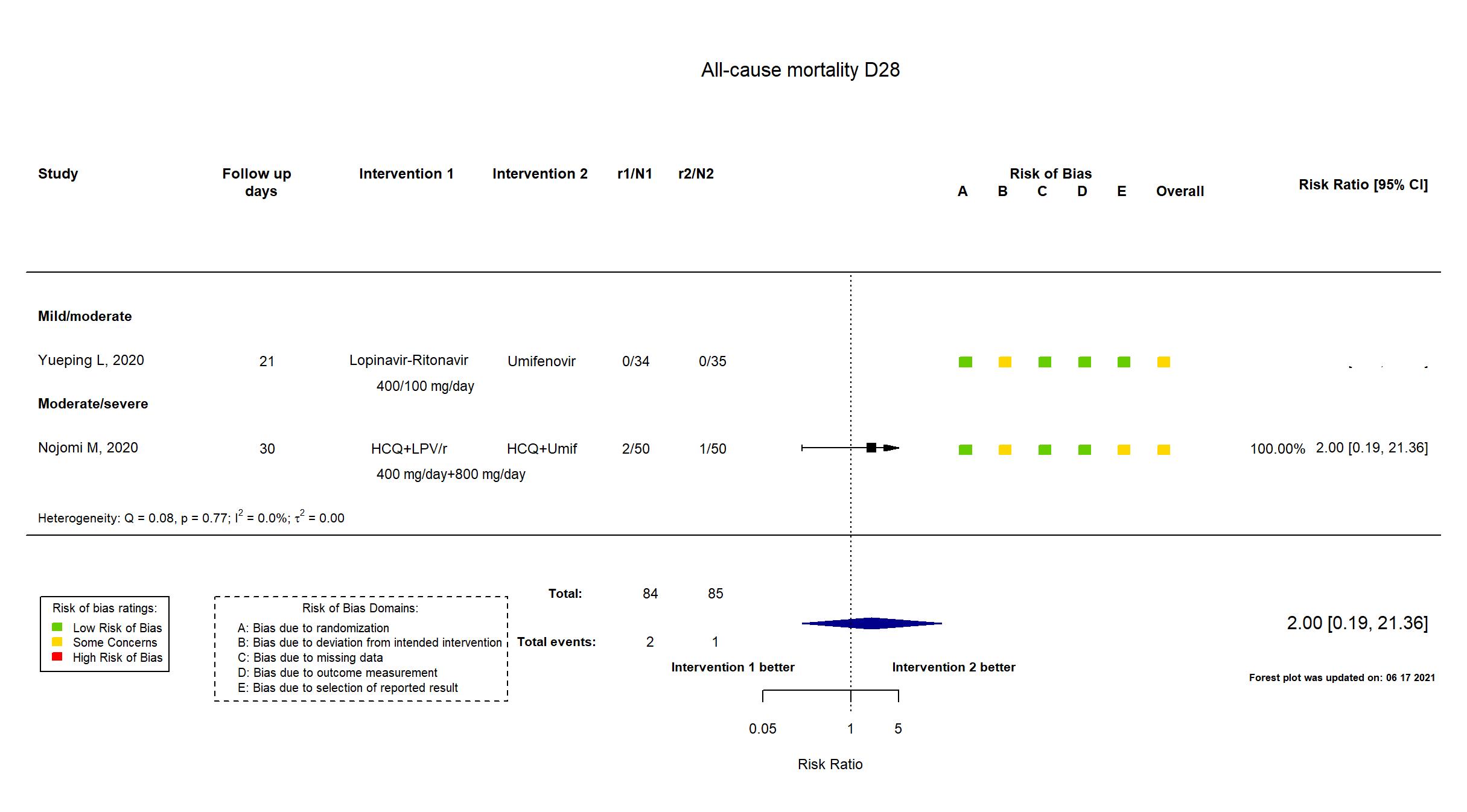
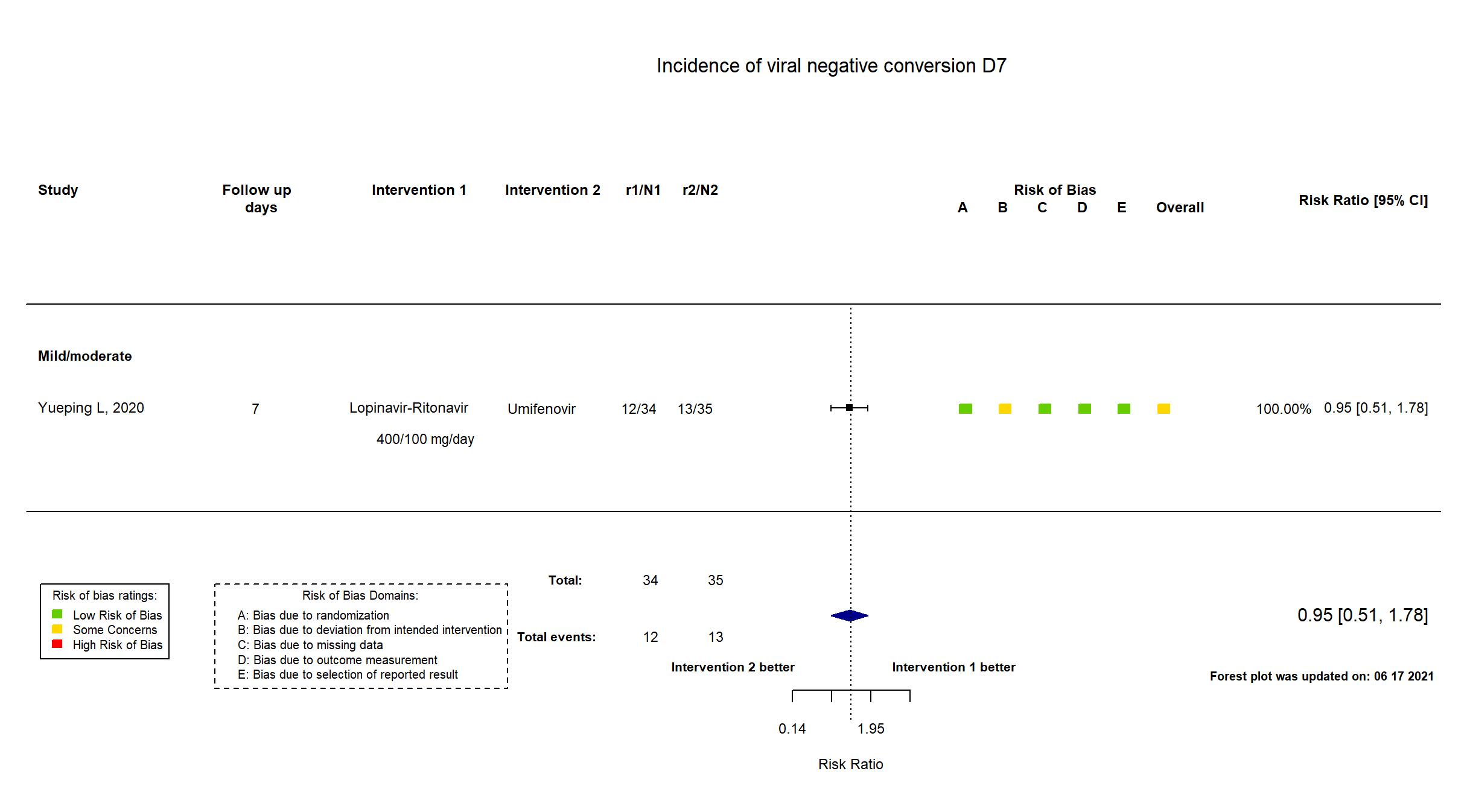
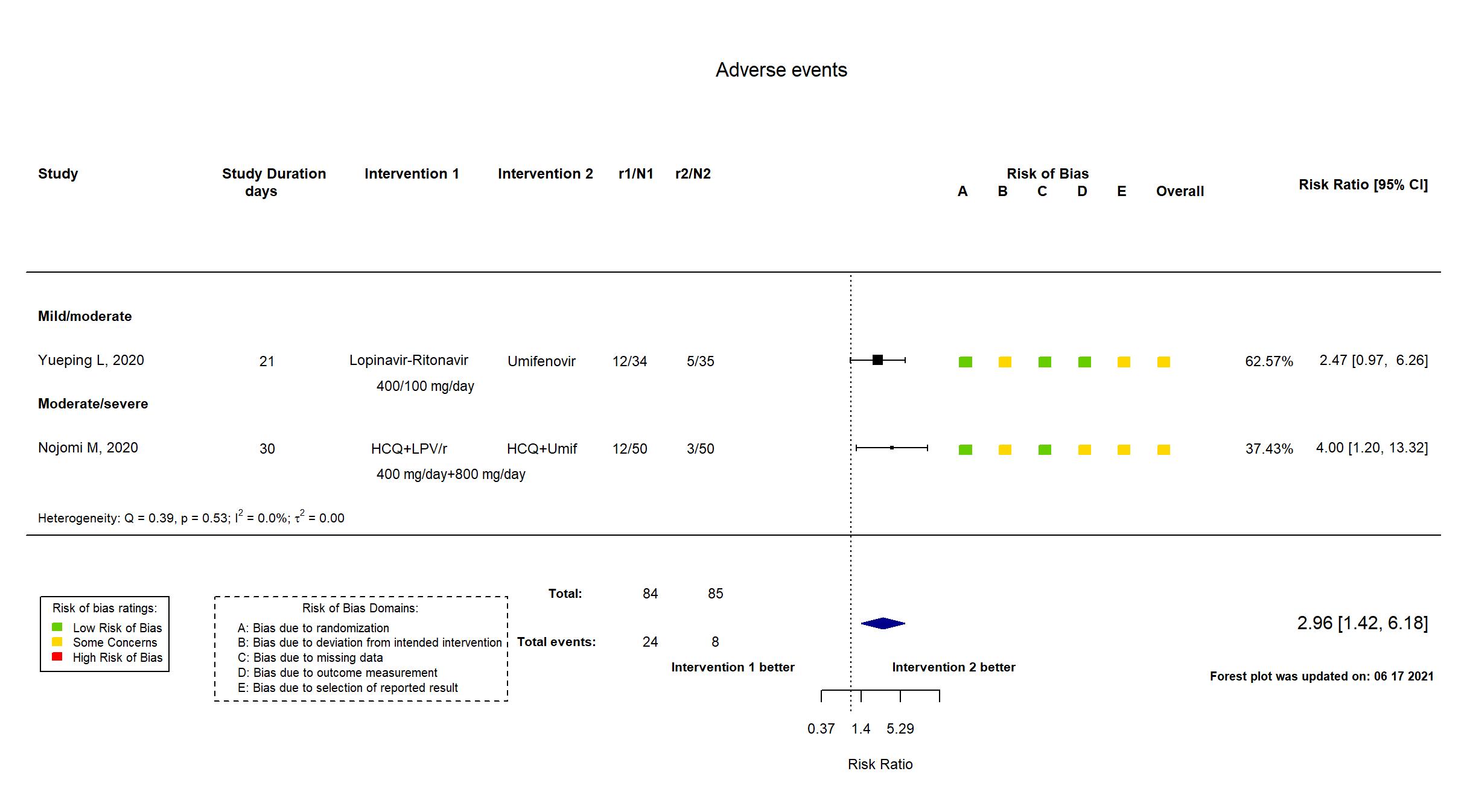
Lopinavir + Ritonavir vs Umifenovir (RCT)
Hospitalized patients
FOREST PLOTS -2021-06-18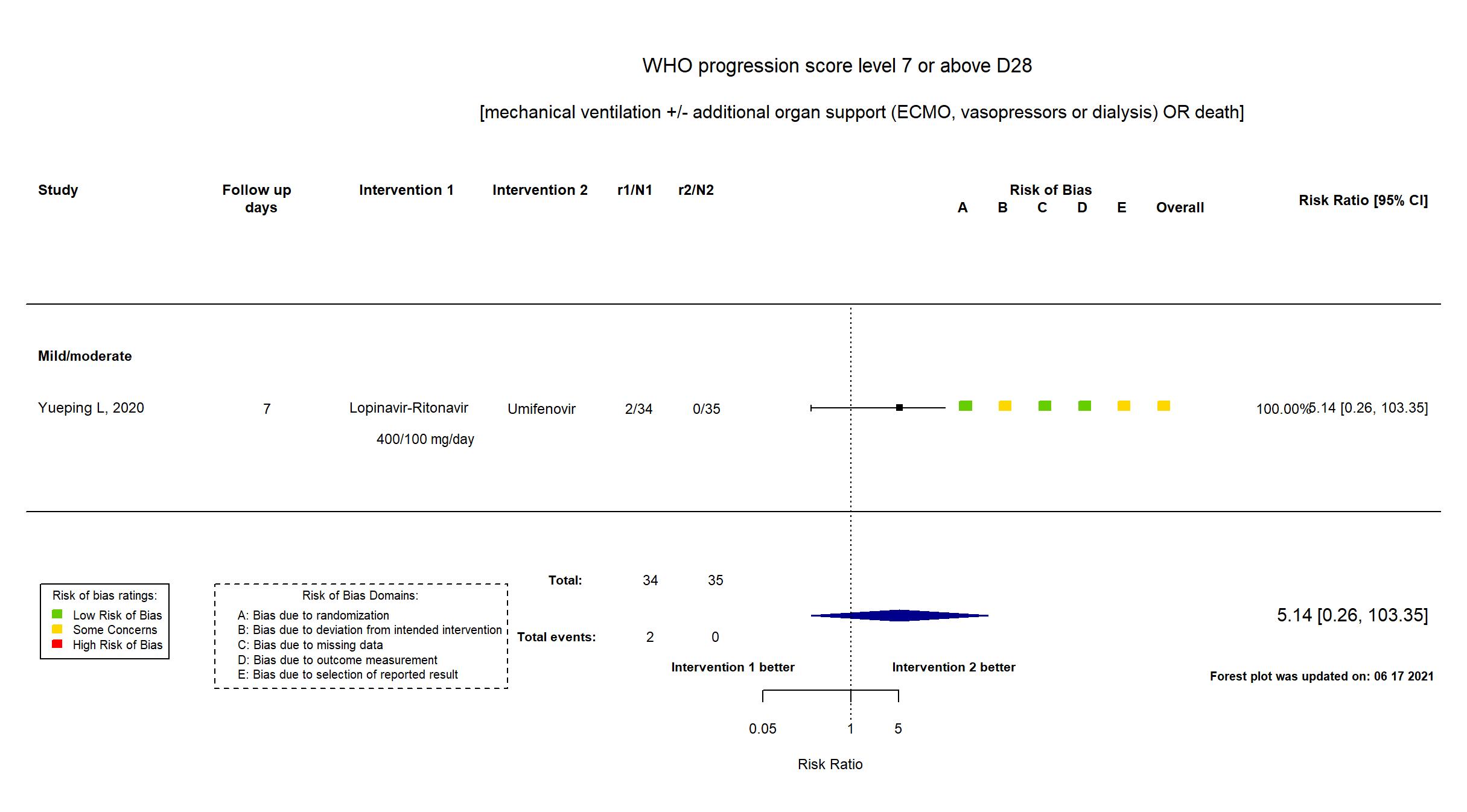





Trial IRCT20180725040596N2
Publication Nojomi M, BMC Infect Dis. (2020) (published paper)
Dates: 2020-04-20 to 2020-06-18
Funding: Public/non profit (Iran University of Medical Sciences)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / Iran Follow-up duration (days): 30 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
HCQ+LPV/r Hydroxychloroquine: 400 mg on first day. Lopinavir/ritonavir: 400 mg twice a day for 7 to 14 days |
|
| Control
HCQ+Umif Hydroxychloroquine: 400 mg twice on first day Umifenovir: 200 mg orally 3 times a day for 7 to 14 days | |
| Participants | |
| Randomized participants : HCQ+Umif=50 HCQ+LPV/r=50 | |
| Characteristics of participants N= 100 Mean age : NR 60 males Severity : Mild: n=* / Moderate: n=* / Severe: n=* Critical: n=0 | |
| Primary outcome | |
| In the register Fever, Complete blood count, C-reactive protein, chest CT Scan view symptoms, Measurement of blood oxygen saturation and no adjuvant oxygen therapy, Hospital admission days (NB - duration of hospitalisation was added as a primary outcome after study | |
| In the report Hospitalization duration and clinical improvement 7 days after admission | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the pre-print article, the study registry was used in data extraction and risk of bias assessment. The target sample size specified in the registry was achieved. Several changes were made to the trial registration. Chest CT scan was added as an outcome during the conduct of the study. After study completion, changes were made to primary and secondary outcomes, including the addition of the primary outcome as reported in the pre-print article. During the conduct of the study the treatments in the trial registration were changed: the dosage of Umifenovir was increased; the duration of both study treatments was extended; the duration of Hydroxychloroquine treatment was extended in the Umifenovir arm but not in the Lopinavir-ritonavir arm. |
Trial NCT04252885
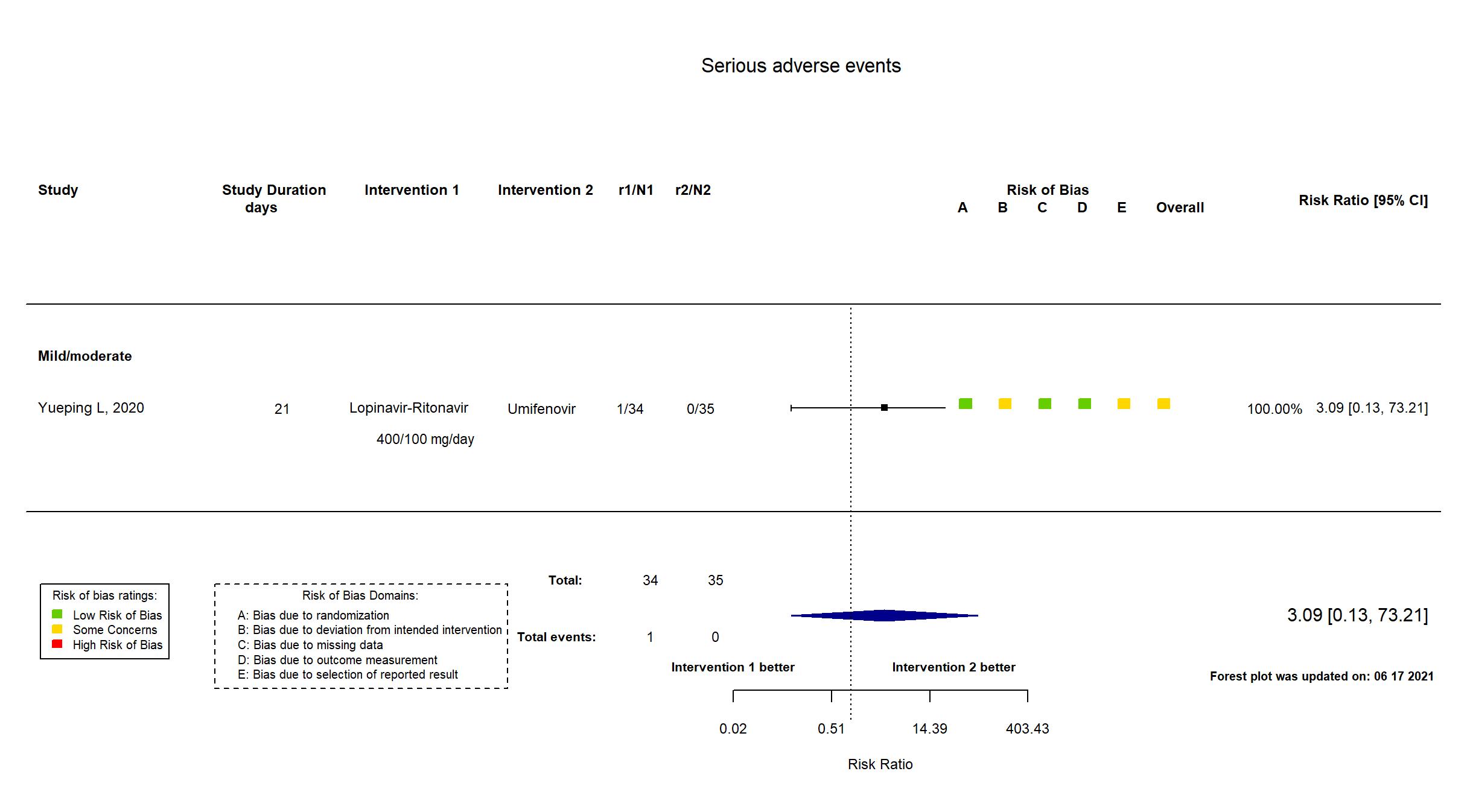
Publication Yueping L, CellPress (2020) (published paper)
Dates: 01feb2020 to 28mar2020
Funding: Public/non profit (Project 2018ZX10302103-002, 2017ZX10202102-003-004 and Infectious Disease Specialty of Guangzhou High-level Clinical Key Specialty (2019-2021) )
Conflict of interest: No
| Methods | |
| RCT Blinding: Participants | |
| Location :
Single center / China Follow-up duration (days): 21 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Lopinavir-Ritonavir LPV/r: 200/50 mg orally twice a day for 7-14 days Umifenovir 200 mg orally 3 times a day for 7-14 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Lopinavir-Ritonavir=34 Umifenovir=35 Standard care=17 | |
| Characteristics of participants N= 86 Mean age : NR 40 males Severity : Mild: n=11 / Moderate: n=75 / Severe: n=0 Critical: n=0 | |
| Primary outcome | |
| In the register The rate of virus inhibition [ Time Frame: Day 0, 2, 4, 7, 10, 14 and 21 ] | |
| In the report Time of positive-to-negative conversion of SARS-CoV-2 nucleic acid from initiating treatment to day 21, with the enrollment day as the first day of treatment | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: No |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to all available versions of the published/pre-print article, the study registry and the reply provided by authors were used in data extraction and risk of bias assessment.The study did not achieve the target sample size specified in the trial registry. There is no change from the trial registration in the intervention and control treatments. The primary outcome indicated in the registry is measured at multiple time points, some of which are not reported in the paper. Some outcomes are reported in the paper, but were not pre-specified in the trial registry/protocol (e.g., adverse events). |