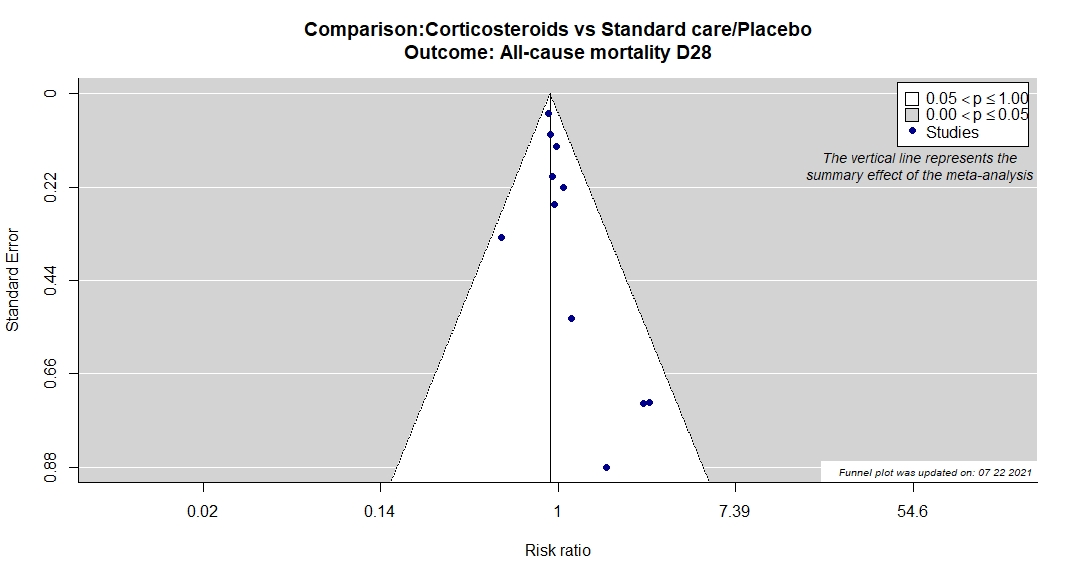
Studies description
Trial NCT02735707
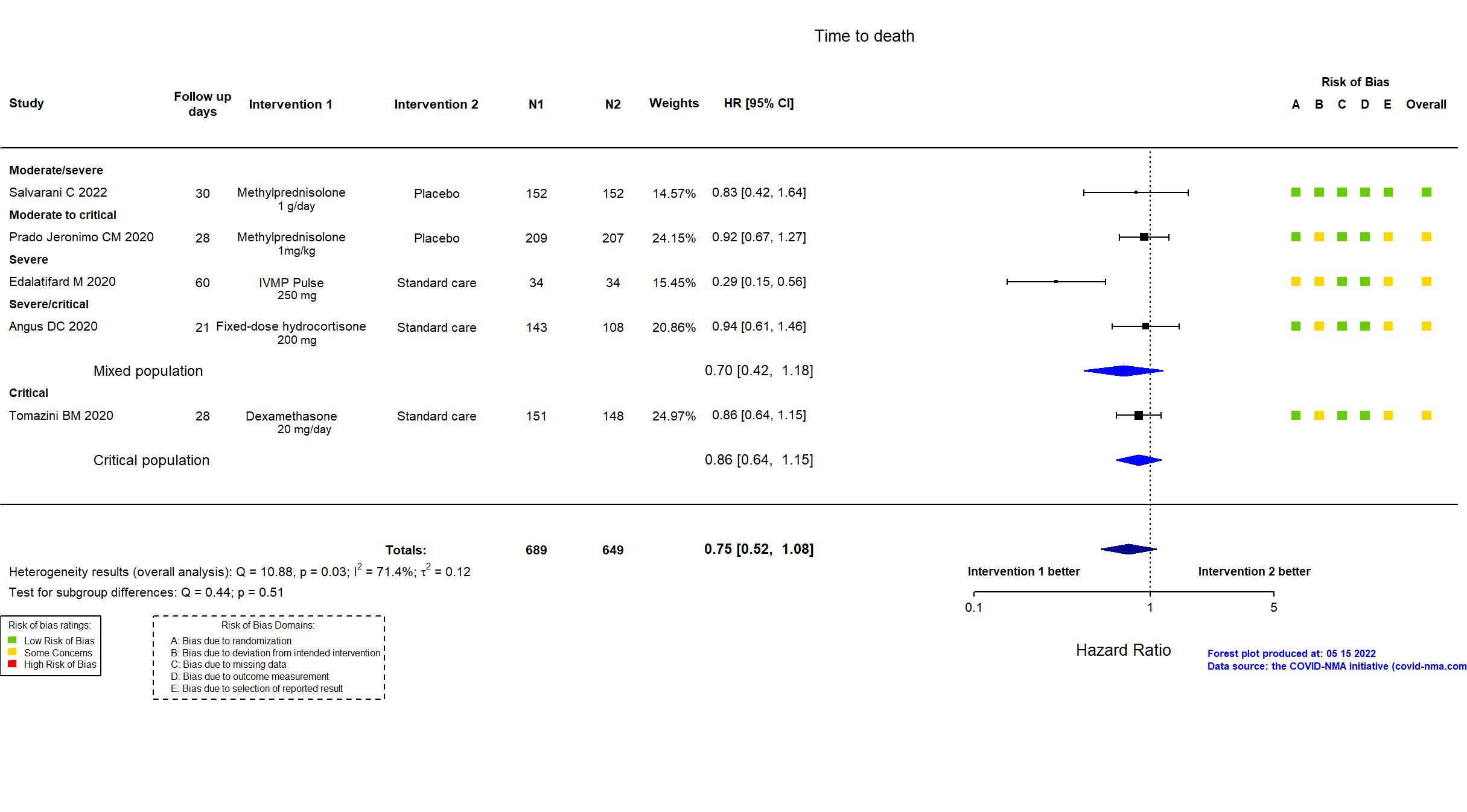
Publication REMAP-CAP - Angus DC, JAMA (2020) (published paper)
Dates: 2020-03-09 to 2020-06-17
Funding: Public/non profit (Platform for European Preparedness Against (Re-) emerging Epidemics (PREPARE) consortium by the European Union, FP7-HEALTH-2013-INNOVATION-1, the Australian National Health and Medical Research Council, the New Zealand H)
Conflict of interest: Yes
Trial EudraCT202000193437
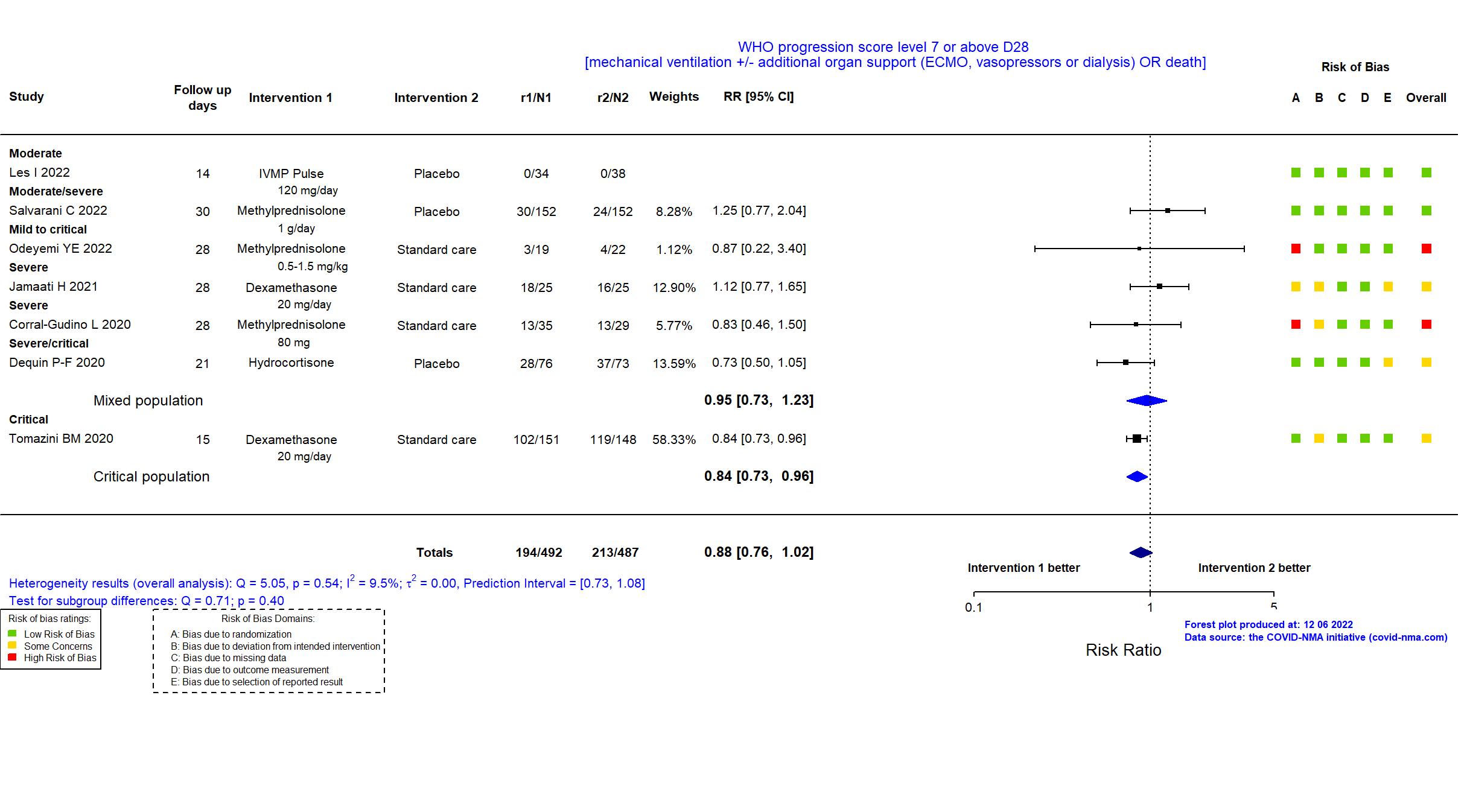
Publication GLUCOCOVID - Corral-Gudino L, medRxiv (2020) (preprint)
Dates: 2020-04-01 to 2020-05-31
Funding: No specific funding (The authors received no specific funding for this work)
Conflict of interest: No
Trial NCT02517489
Publication Dequin P-F, JAMA (2020) (published paper)
Dates: 2020-03-07 to 2020-06-01
Funding: Public/non profit (French Ministry of Health, Programme Hospitalier de Recherche Clinique (PHRC))
Conflict of interest: Yes
Trial NCT04244591
Publication Steroids-SARI - Du, Unpublished (2020) ( )
Funding: Public/non profit (Peking Union Medical College Hospital, Zhongda Hospital, Zhongnan Hospital, Renmin Hospital of Wuhan University)
Conflict of interest: *
Trial IRCT20200404046947N1
Publication Edalatifard M, Eur Respir J (2020) (published paper)
Dates: 2020-04-20 to 2020-06-20
Funding: Public/non profit (Deputy of Research, Tehran University of Medical Sciences)
Conflict of interest: No
Trial IRCT20200406046963N1
Publication Farahani R, Research Square (2020) (preprint)
Dates: 2020-04-01 to 2020-05-30
Funding: Public/non profit (AJA University of Medical Science)
Conflict of interest: No
Trial NCT04381936
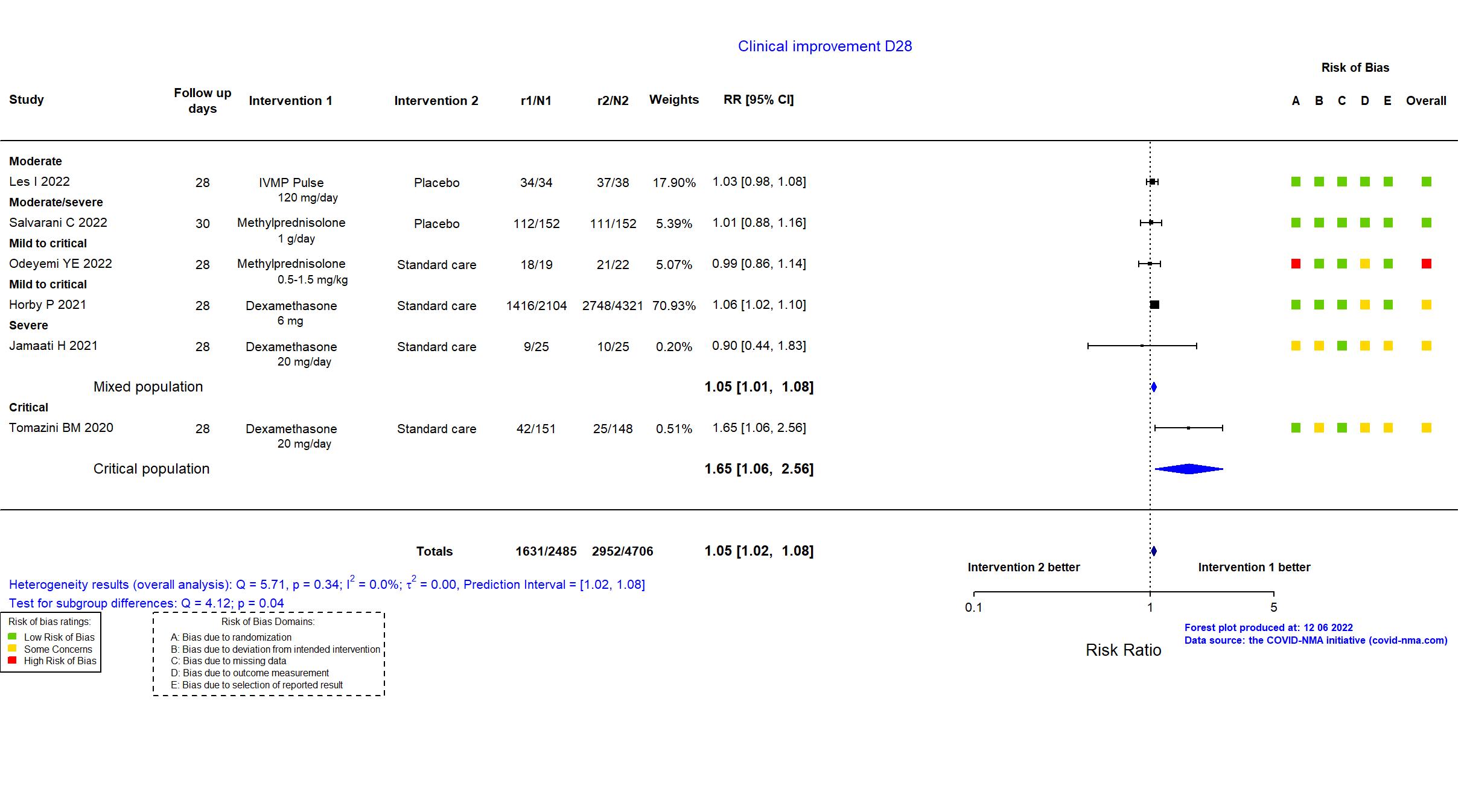
Publication RECOVERY (Dex) - Horby P, N Engl J Med (2021) (published paper)
Dates: 2020-03-19 to 2020-06-08
Funding: Mixed (University of Oxford from UK Research and Innovation/National Institute for Health Research (NIHR); NIHR Oxford Biomedical Research Centre; Wellcome; Bill and Melinda Gates Foundation; Department for International Development; Health Data Research UK)
Conflict of interest: No
Trial IRCT20151227025726N17
Publication Jamaati H, Eur J Pharmacol (2021) (published paper)
Funding: Public/non profit (Shahid Beheshti University of Medical Sciences)
Conflict of interest: No
Trial NCT04438980 ; EudraCT 2020-001827-15
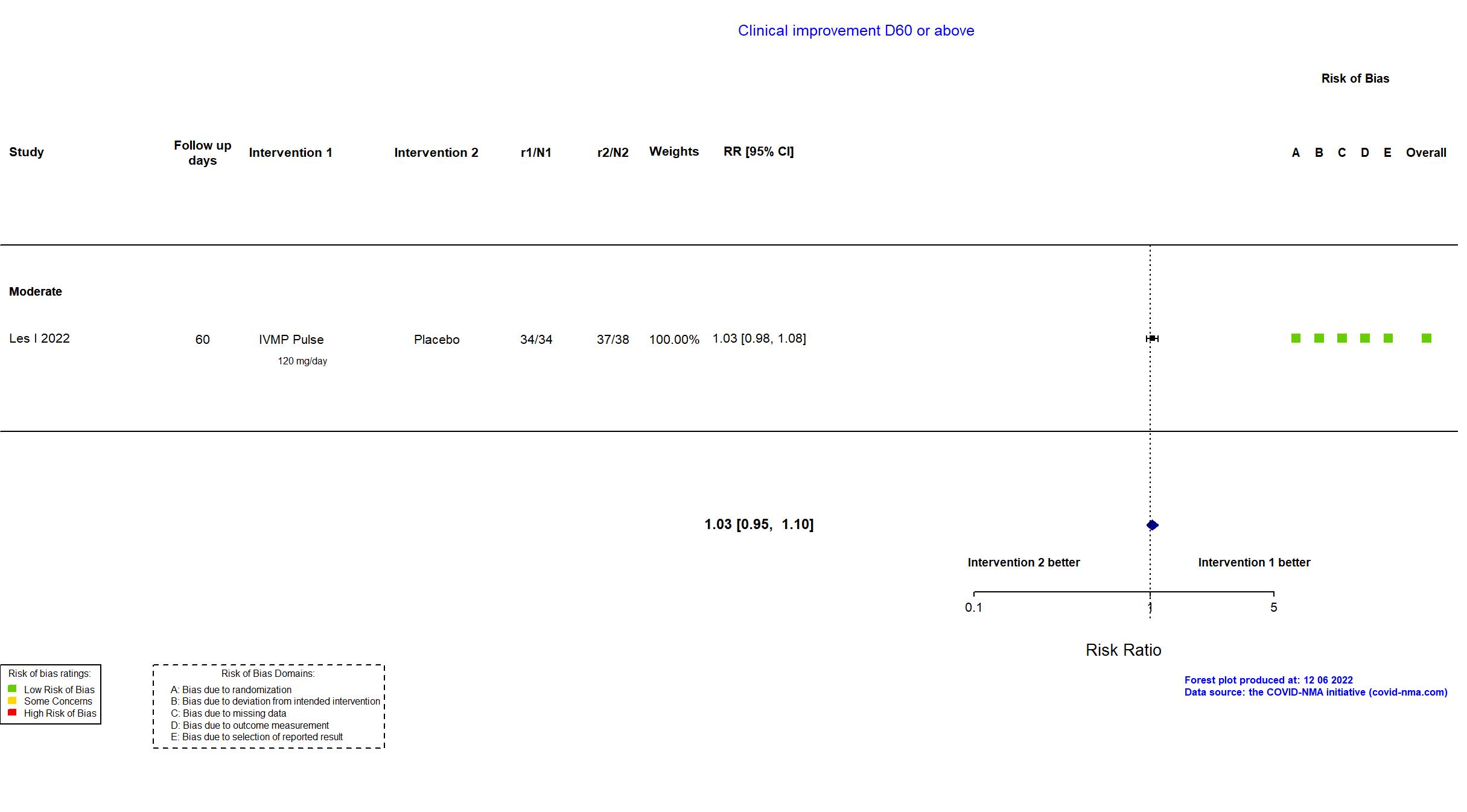
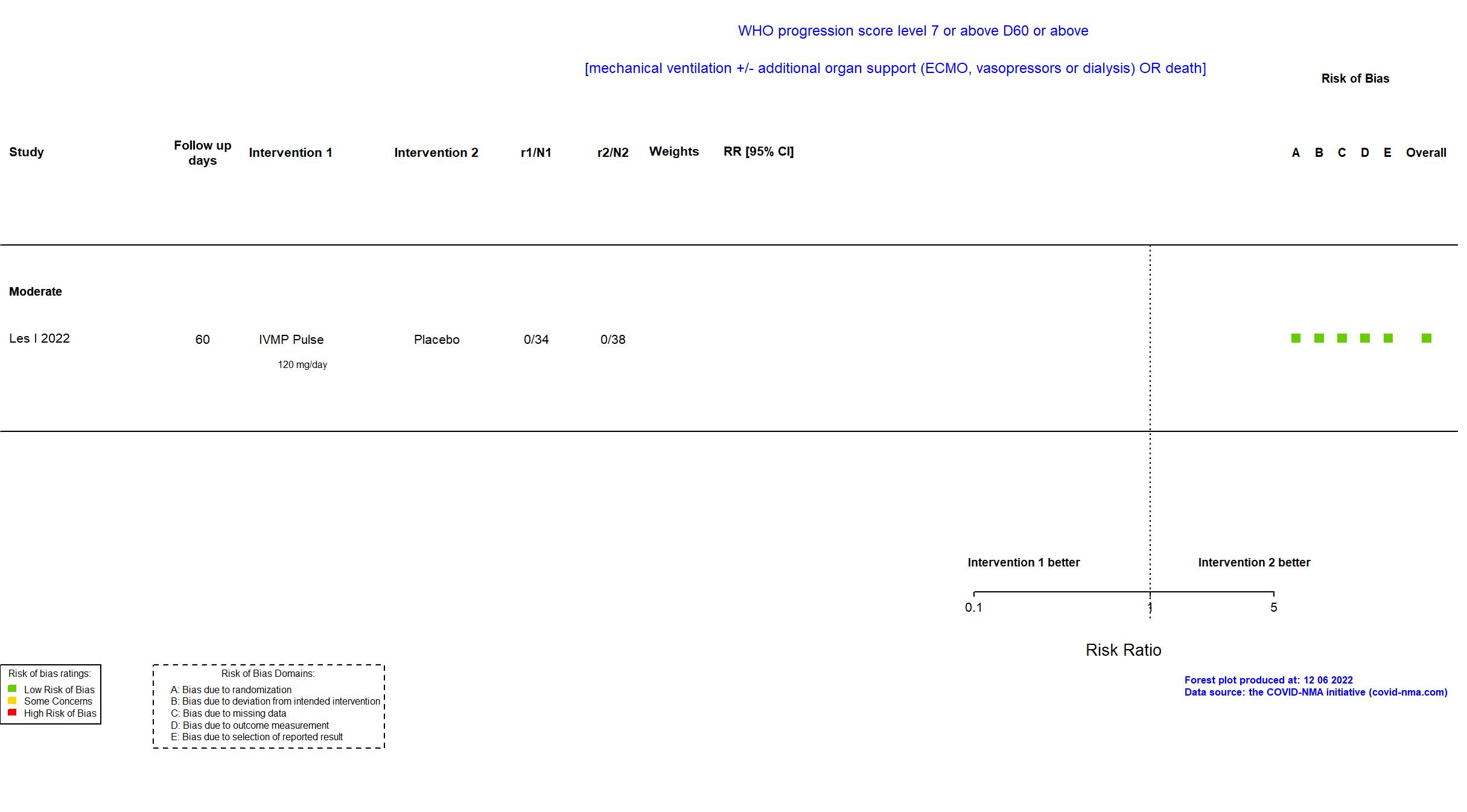
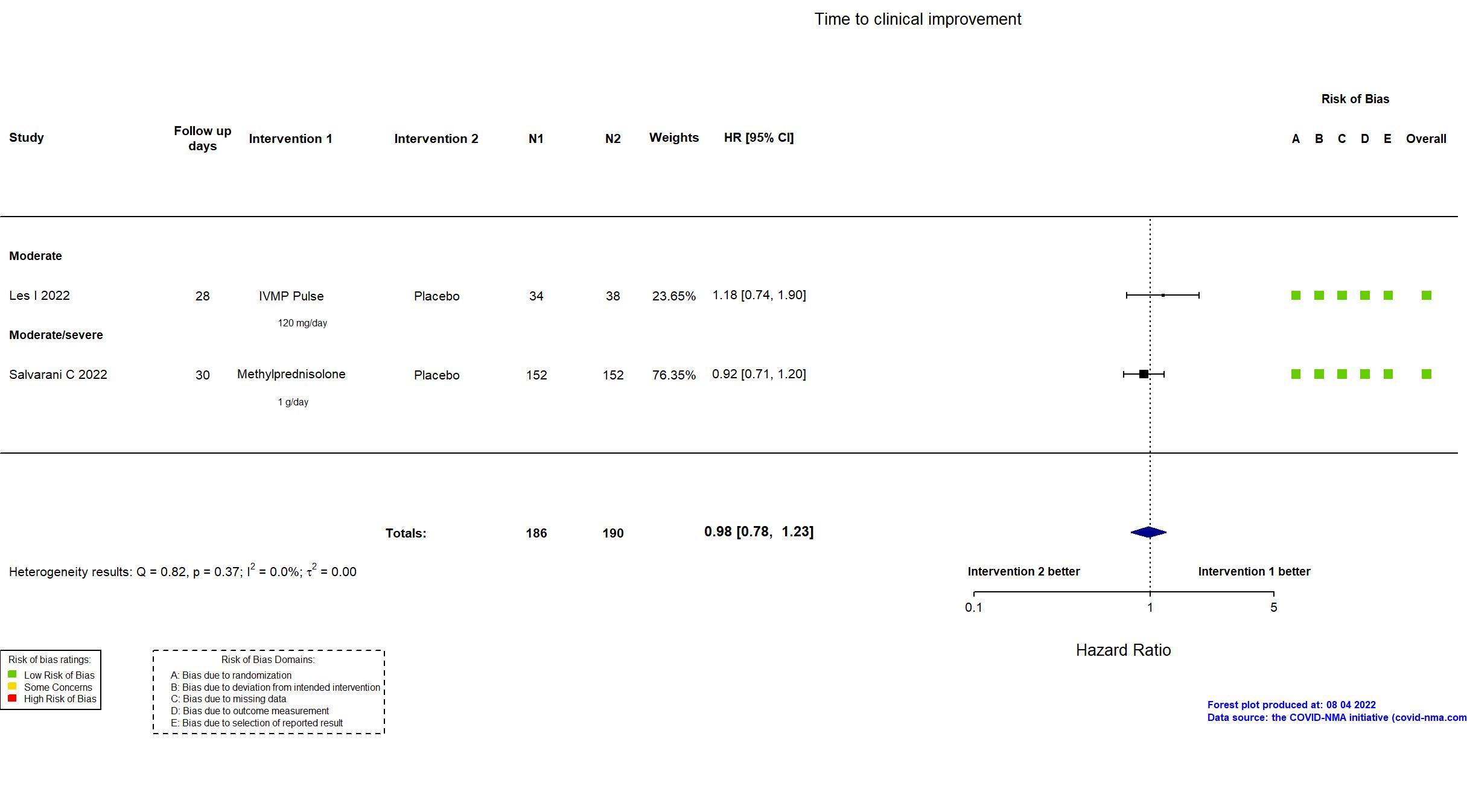
Publication CORTIVID - Les I, Front Med (2022) (published paper)
Dates: 2020-05-08 to 2021-03-13
Funding: Public/non profit (ISCIII - Instituto de Salud Carlos III (Carlos III Health Institute)
)
Conflict of interest: No
Trial NCT04348305
Publication COVID STEROID - Munch MW, Acta Anaesthesiol Sc (2021) (published paper)
Dates: 2020-04-17 to 2020-06-16
Funding: Mixed (Novo Nordisk Foundation, Rigshospitalet's Research Council, and Pfizer.)
Conflict of interest: No
Trial NCT03852537
Publication SMART Trial - Odeyemi YE, Crit Care (2022) (published paper)
Dates: 2020-03-01 to 2020-11-30
Funding: Public/non profit (The American Heart Association COVID-19 Rapid Response Grant. The Mayo Clinic Critical Care Independent Multidisciplinary Program (IMP) Research Grant (Institutional Departmental grant). National Center for Advancing Translational Science (NCATS). National Heart, Lung, and Blood Institute)
Conflict of interest: No
Trial NCT04343729
Publication Prado Jeronimo CM, Clin Infect Dis (2020) (published paper)
Dates: 2020-04-08 to 2020-06-16
Funding: Public/non profit (Superintendencia da Zona Franca de Manaus, Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior, Departamento de Ciencia e Tecnologia/Ministerio da Saude, Ministerio da Ciencia, Tecnologia e Inovacoes, Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, Fundacao de Amparo a Pesquisa do Estado do Amazonas)
Conflict of interest: No
Trial NCT04673162 ; EudraCT 2020-004323-16
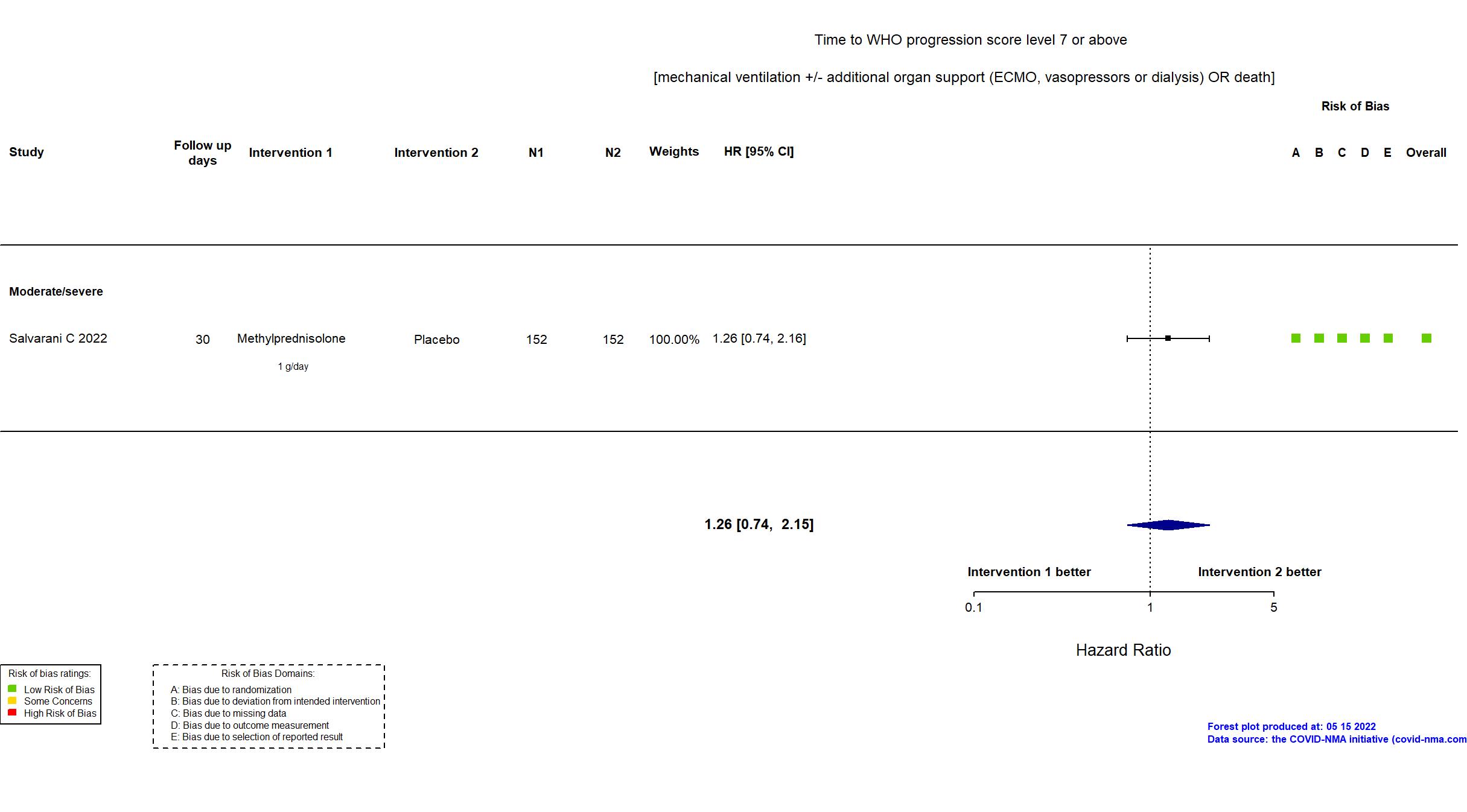
Publication Salvarani C, Eur Respir J (2022) (published paper)
Dates: 2020-12-21 to 2021-03-10
Funding: No specific funding (This research received no external funding. The coordinator centre and all participating centres are using local resources to conduct the trial.)
Conflict of interest: No
Trial NCT04273321
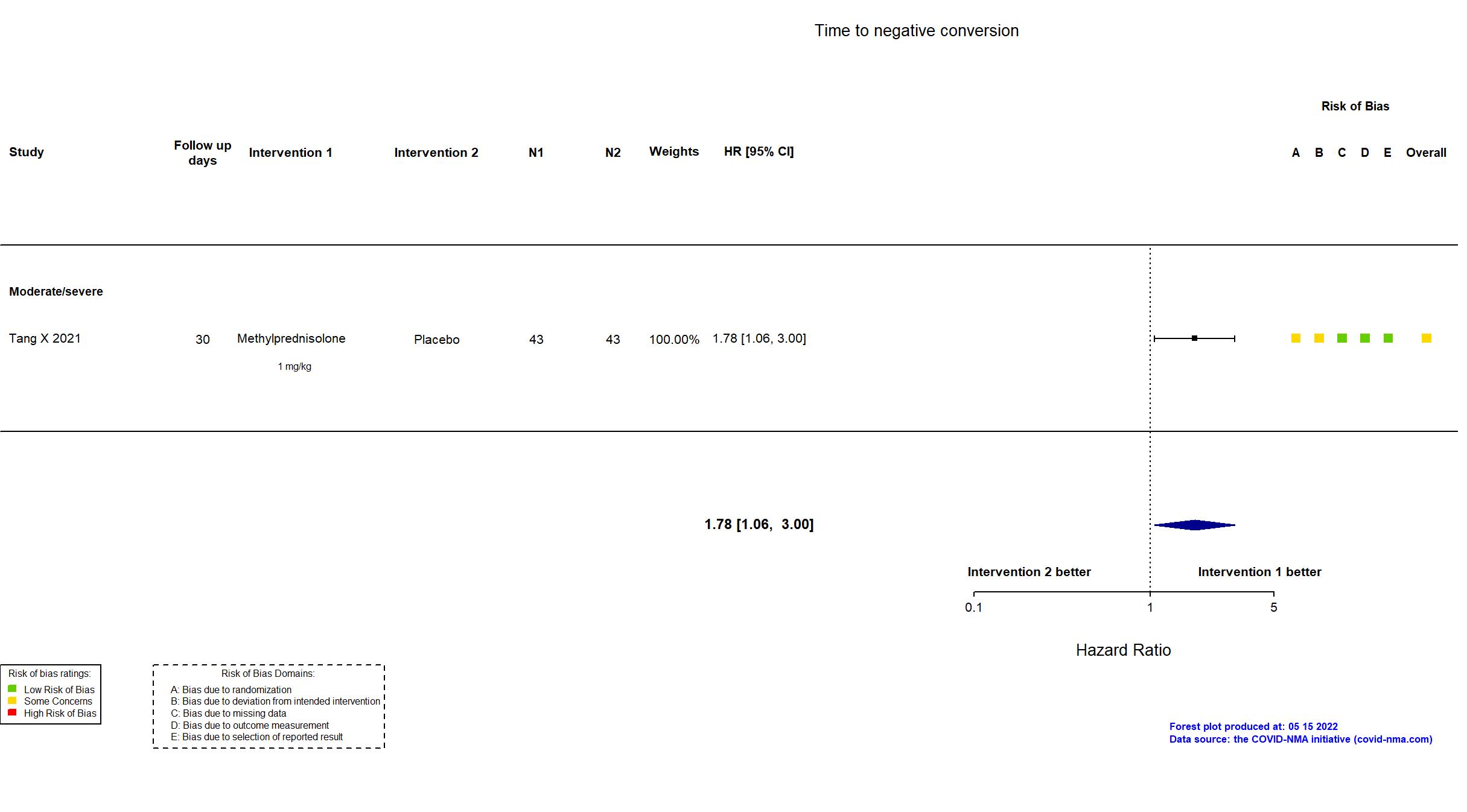
Publication Tang X, Respiration (2021) (published paper)
Dates: 2020-02-19 to 2020-03-31
Funding: Public/non profit (Beijing Municipal Admin of Hospitals’ Mission Plan; Excellence Prog of Beijing Clin Key Specialty; Novel Coronavirus Pneumonia Key Tech R&D Funding of Beijing Municipal Admin of Hospitals)
Conflict of interest: No
Trial NCT04327401
Publication Tomazini BM, JAMA (2020) (published paper)
Dates: 2020-04-17 to 2020-06-23
Funding: Mixed (Coalition COVID-19 Brazil; Laboratorios Farmaceuticos)
Conflict of interest: Yes
Trial NCT04325061
Publication DEXA-COVID19 - Villar J, Unpublished (2020) ( )
Funding: Public/non profit (Instituto de Salud Carlos III, Madrid, Spain(CB06/06/1088, PI19/00141); Asociación Científica Pulmón y VentilaciónMecánica, Las Palmas de Gran Canaria, Spain; and the Canadian Institute for Health Research, Ottawa, Canad)
Conflict of interest: No