Baricitinib vs Standard care/Placebo (RCT)
Hospitalized patients
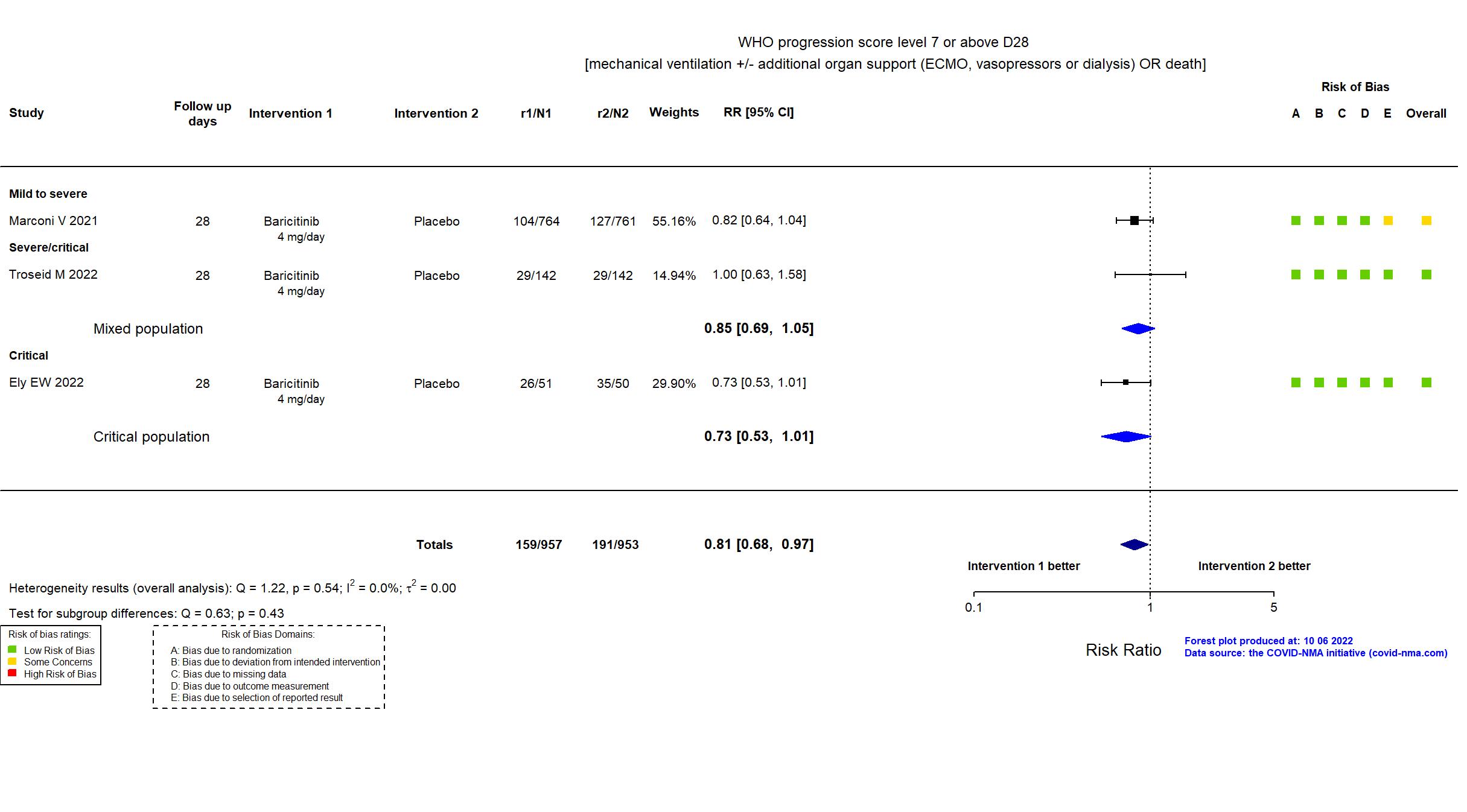
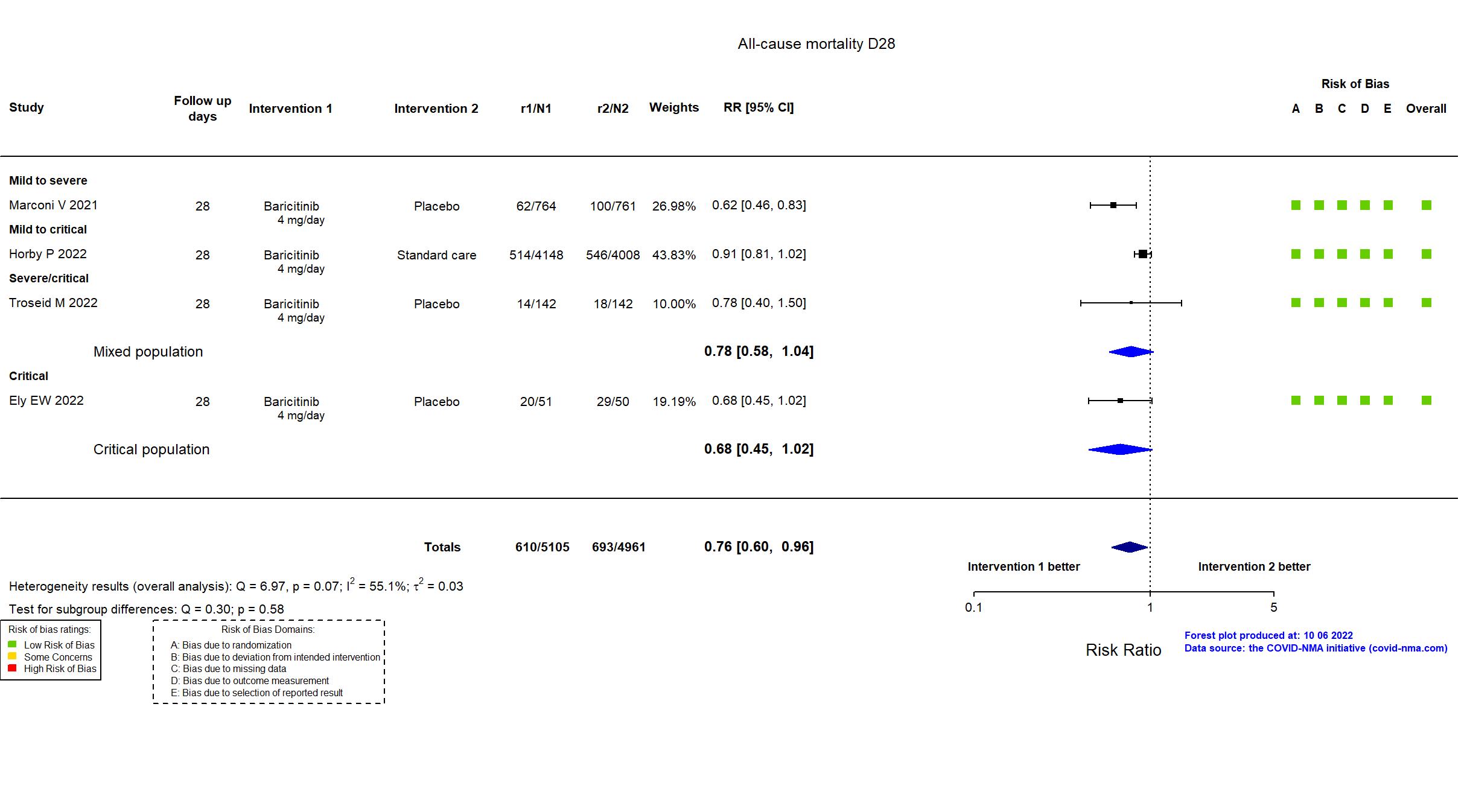
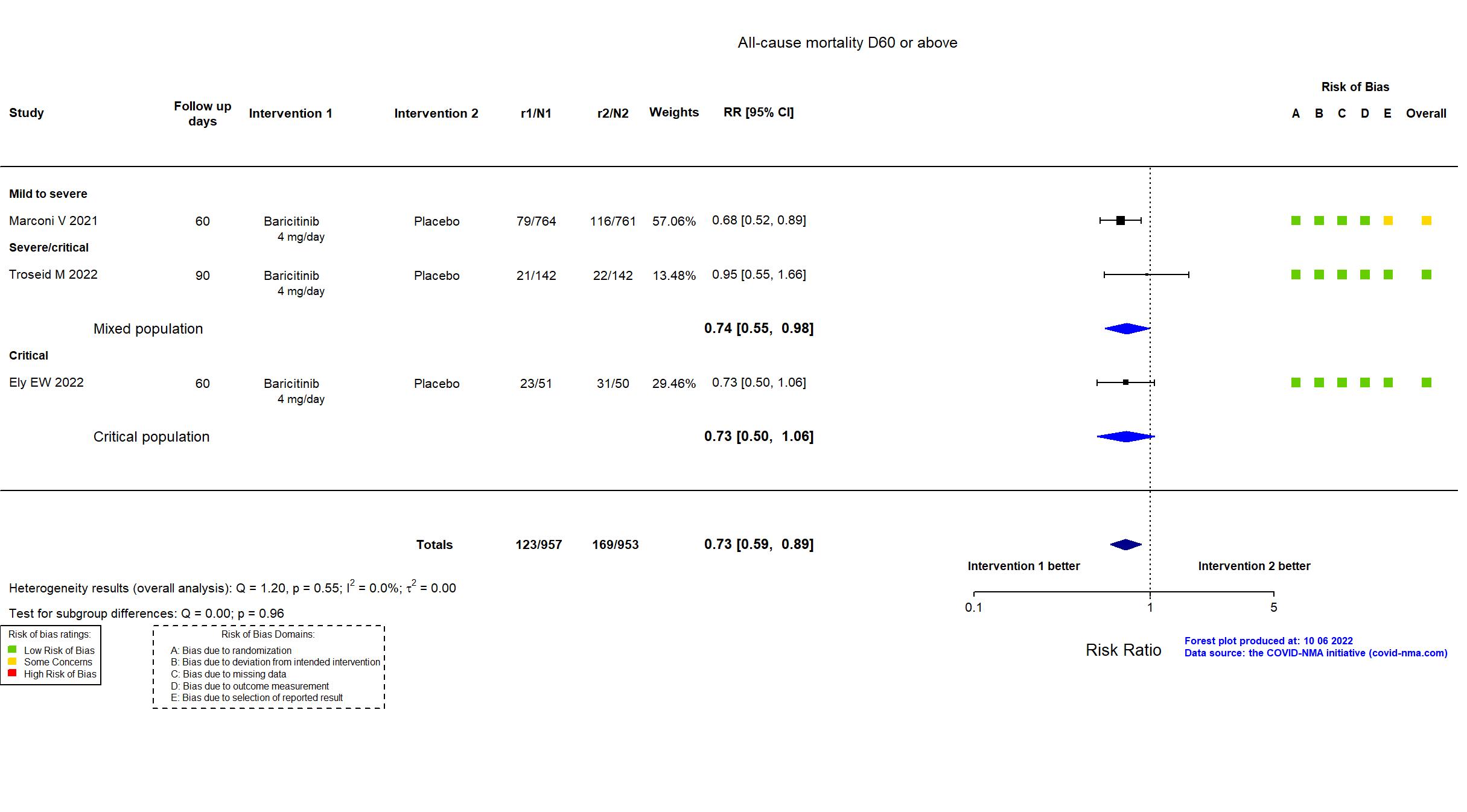
FOREST PLOTS -2022-10-07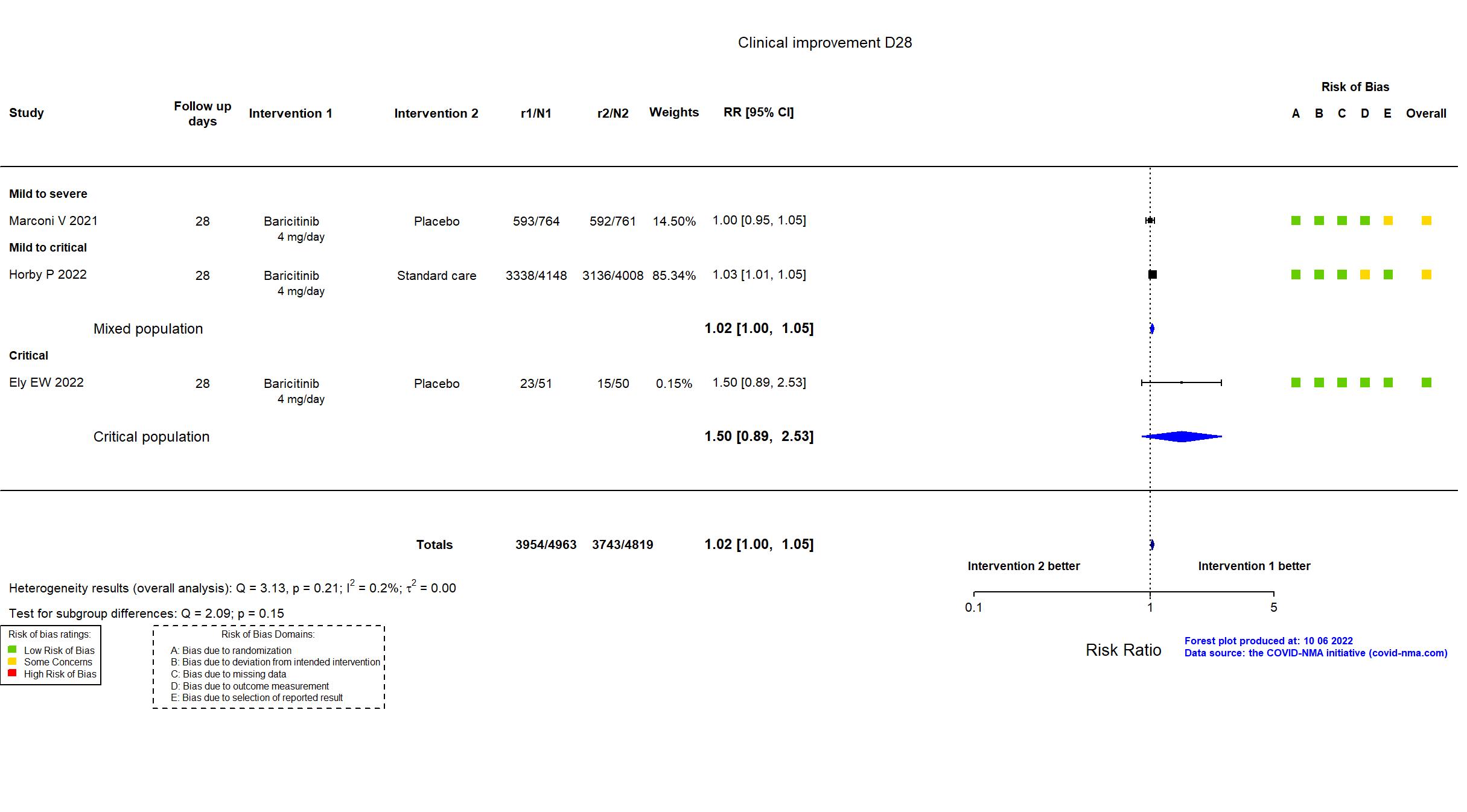

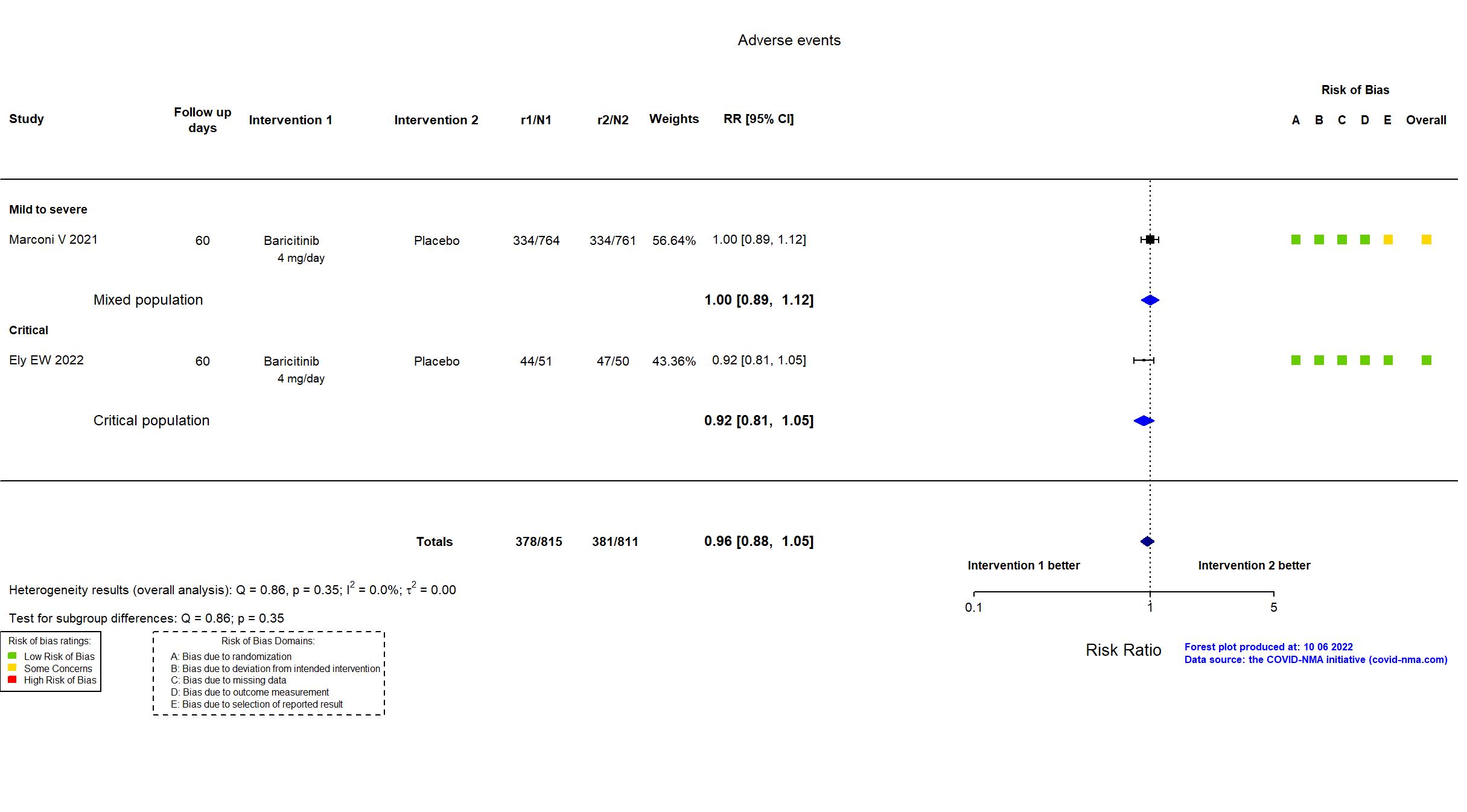
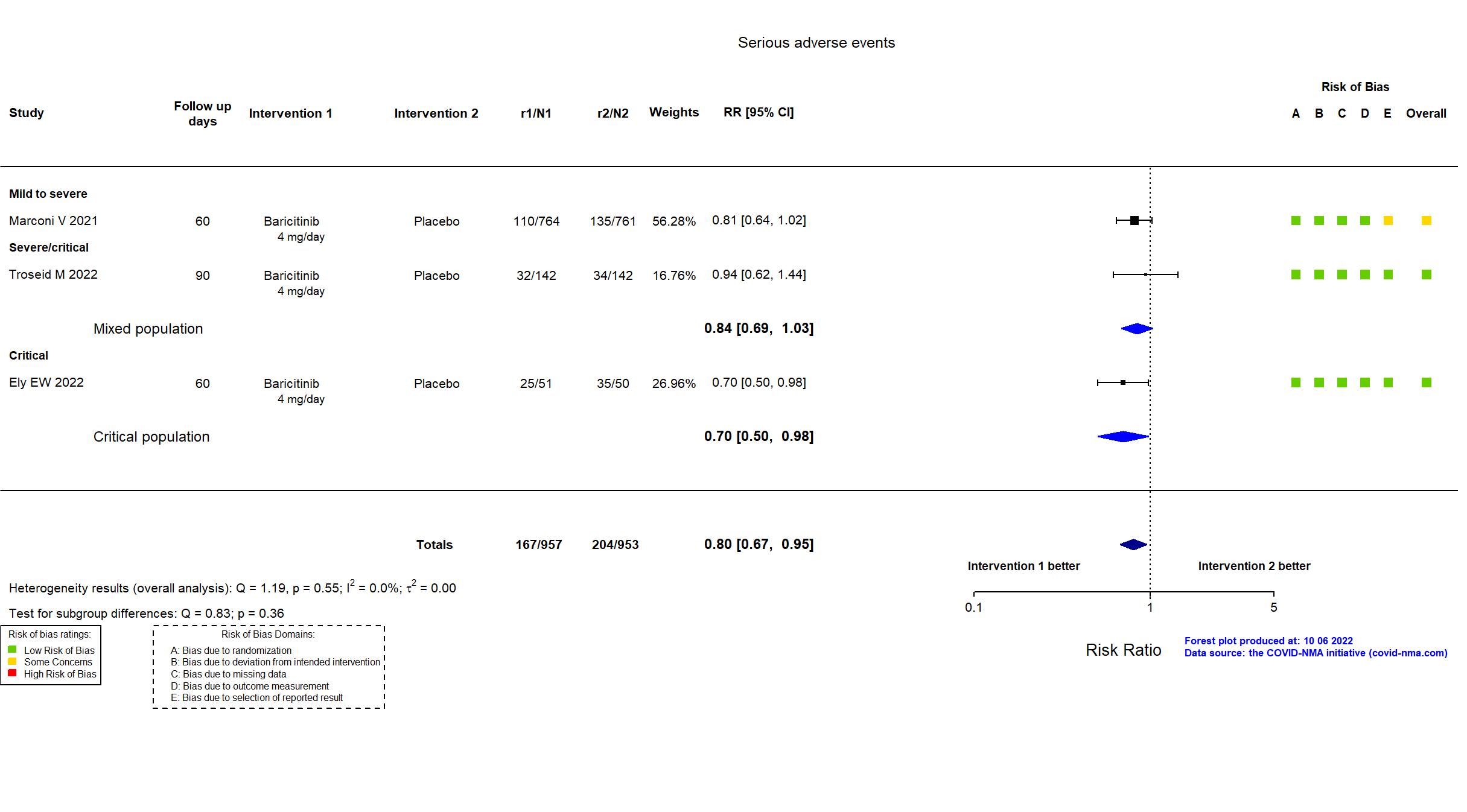
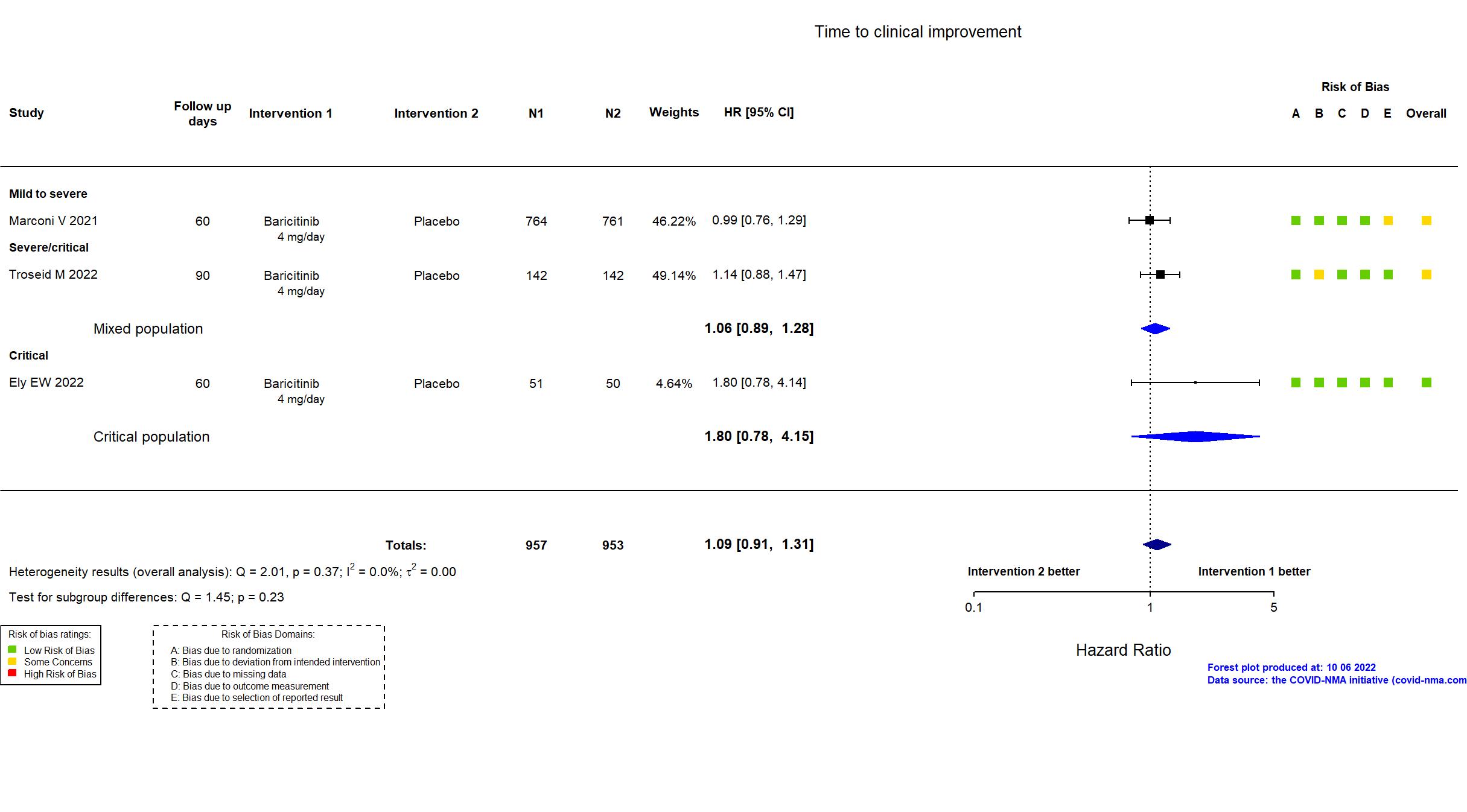
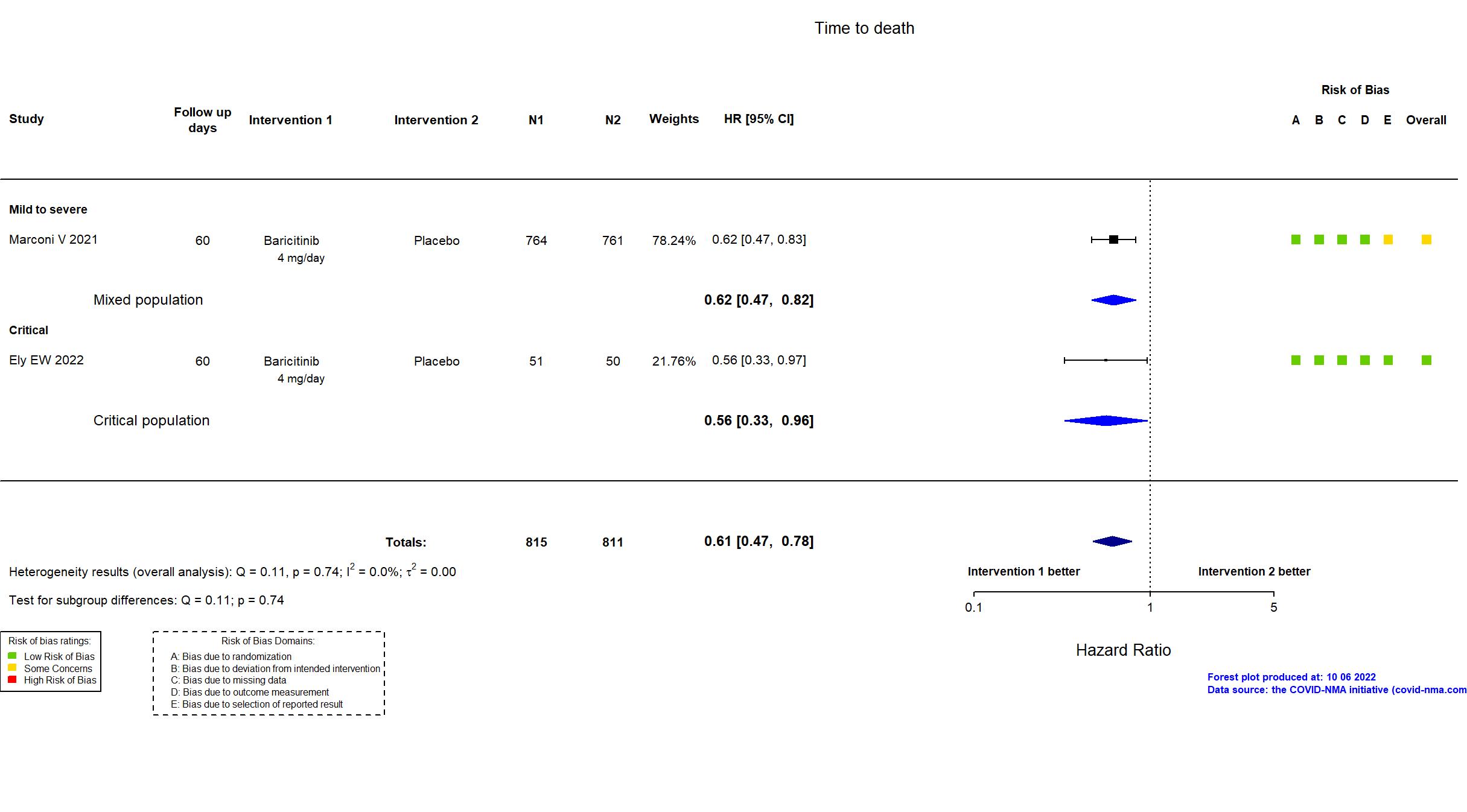









Trial NCT04421027
Publication COV-BARRIER - Ely EW, Lancet Respir Med (2022) (published paper)
Dates: 2020-12-23 to 2021-04-10
Funding: Private (Eli Lilly and Company)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / Argentina, Brazil, Mexico, USA Follow-up duration (days): 60 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Baricitinib 4 mg by nasogastric tube or orally daily up to 14 days or until discharge. If eGFR = ≥30 to <60 mL/min/1·73 m2 at baseline or at any time during treatment, 2-mg daily until eGFR = ≥60 mL/min/1·73 m2. |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Placebo=50 Baricitinib=51 | |
| Characteristics of participants N= 101 Mean age : NR 55 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=0 Critical: n=101 | |
| Primary outcome | |
| In the register Percentage of Participants who Die or Require Non-Invasive Ventilation/High-Flow Oxygen or Invasive Mechanical Ventilation (including extracorporeal membrane oxygenation [ECMO]) [ Time Frame: Day 1 to Day 28 ] | |
| In the report All-cause mortality by day 28 and day 60; number of ventilator-free days; overall improvement (assessed by odds of improvement in clinical status) on National Institute of Allergy and Infectious Disease Ordinal Scale (NIAID-OS) evaluated at days 4, 7, 10, 14, and 28; proportion of participants with at least 1-point improvement on the NIAID-OS or live discharge from the hospital at days 4, 7, 10, 14, and 28; duration of hospitalisation; and time to recovery through day 28. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Low |
| General comment |
In addition to the published and pre-print article, the registry, addendum protocol, original trial protocol with statistical analysis plan and supplementary appendices were used in data extraction and assessment of risk of bias. The COV-BARRIER addendum trial in COVID-19 patients requiring invasive mechanical ventilation or ECMO was an extension of the original COV-BARRIER trial, in which eligible patients did not require either. For this reason, the inclusion and exclusion criteria do not wholly reflect those in the registry. Likewise, the outcomes reported do not reflect the primary outcome in the overall trial registry (need for IVM or ECMO or death), but do reflect the other registry outcomes. Safety outcomes reported were not in the registry but were in the original protocol. The study achieved its target sample size.
The study was updated on March 17th, 2022 with data from the published report. |
Trial NCT04381936; EudraCT2020-001113-21; ISRCTN50189673
Publication RECOVERY - Horby P, Lancet (2022) (published paper)
Dates: 2021-02-02 to 2021-12-29
Funding: Public/non profit (UK Research and Innovation (Medical Research Council) and National Institute of Health Research)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / UK Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Baricitinib 4 mg orally per day for 10 days or discharge (reduced for patients with impaired renal function or children <9 years) |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Baricitinib=4148 Standard care=4008 | |
| Characteristics of participants N= 8156 Mean age : NR 5378 males Severity : Mild: n=465 / Moderate: n=5513 / Severe: n=1927 Critical: n=251 | |
| Primary outcome | |
| In the register All-cause mortality [ Time Frame: Within 28 days after randomisation ] | |
| In the report 28-day all-cause mortality | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the pre-print article, the registry, protocol, statistical analysis plan and supplementary appendices were used in data extraction and assessment of risk of bias. The primary outcome in the article reflects the primary outcome in the registry and protocol. Recruitment to the trial was terminated after a planned interim analysis on the decision of the Trial Steering Committee when a statistically significant reduction in all-cause mortality was detected.
This study was updated on August 18th, 2022 with data extracted from the published report. |
Trial NCT04421027
Publication COV-BARRIER - Marconi V, Lancet Respir Med (2021) (published paper)
Dates: 2020-06-11 to 2021-01-15
Funding: Private (Eli Lilly and Company (Incyte Corporation licence))
Conflict of interest: Yes
| Methods | |
| RCT Blinding: single blinding | |
| Location :
Multicenter / Argentina, Brazil, Germany, India, Italy, Japan, Mexico, Russia, South Korea, Spain, UK, USA (including Puerto Rico) Follow-up duration (days): 60 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Baricitinib 4 mg orally once daily for 14 days or until discharge from hospital |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Placebo=761 Baricitinib=764 | |
| Characteristics of participants N= 1525 Mean age : NR 963 males Severity : Mild: n=186 / Moderate: n=962 / Severe: n=370 Critical: n=0 | |
| Primary outcome | |
| In the register Percentage of Participants who Die or Require Non-Invasive Ventilation/High-Flow Oxygen or Invasive Mechanical Ventilation (including extracorporeal membrane oxygenation [ECMO]) [ Time Frame: Day 1 to Day 28 ] | |
| In the report Proportion of participants who progressed to high-flow oxygen or non-invasive ventilation, invasive mechanical ventilation or extracorporeal membrane oxygenation, or death by day 28 | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published journal and pre-print articles, the prospective study registry was used in data extraction and risk of bias assessment. The protocol and statistical analysis plan were not accessed at the time of data extraction but they are available from the investigators upon request. The study achieved the target sample size specified in the trial registry. There is no change from the trial registration in the intervention and control treatments. The registry primary outcome reflects the reported primary outcome. The study reported on some secondary outcomes that were not listed in the registry, including adverse events and '≥2-point improvement on NIAID-OS or live discharge from hospital' (used for clinical improvement in our analysis). Some outcomes prespecified are not reported in the paper (e.g., Time to Definitive Extubation).
This trial was updated on September 28th, 2021 with data from the peer-reviewed journal publication. Mortality results were taken from the report and not the registry. |
Trial NCT04891133; EudraCT 2021-000541-41; EU CTIS 2022-500385-99-00
Publication Bari-SolidAct - Troseid M, SSRN (2022) (preprint)
Dates: 2021-06-03 to 2022-03-07
Funding: Mixed (European Commission; EU-SolidAct is part of the European pandemic preparedness network EU RESPONSE, funded by the EU Horizon 2020 Research and Innovation Programme. EU-SolidAct has also received funding from CAPNET (France) and Klinbeforsk (Norway). The baricitinib drug and matching placebo were provided by Eli Lilly and Company. )
Conflict of interest: Yes
| Methods | |
| RCT Blinding: triple blinding | |
| Location :
Multicenter / Austria, Belgium, France, Ireland, Italy, Luxembourg, Norway, Portugal, Spain Follow-up duration (days): 90 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Baricitinib 4 mg orally once a day for up to 14 days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Placebo=142 Baricitinib=142 | |
| Characteristics of participants N= 284 Mean age : NR 211 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=236 Critical: n=39 | |
| Primary outcome | |
| In the register Occurrence of death within 60 days (primary end point, EU SolidAct part B) [ Time Frame: 60 days ] | |
| In the report Occurrence of death within 60 days (measured on day 61 after inclusion). | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the preprint article, the prospective trial registry was used in data extraction and assessment of risk of bias. Neither protocol nor statistical analysis plan were available. The supplementary appendices referred to in the article were not accessible with the preprint version. There is no change from the trial registration in the intervention and control treatments. The primary and secondary outcomes in the article reflect those in the registry. The trial (n = 275) did not achieve its target sample size (n = 1900) because the trial was stopped before reaching the planned sample size due to external evidence from the Recovery trial indicating survival benefit of baricitinib in the trial population. |