Camostat Mesilate vs Placebo (RCT)
Mild outpatients
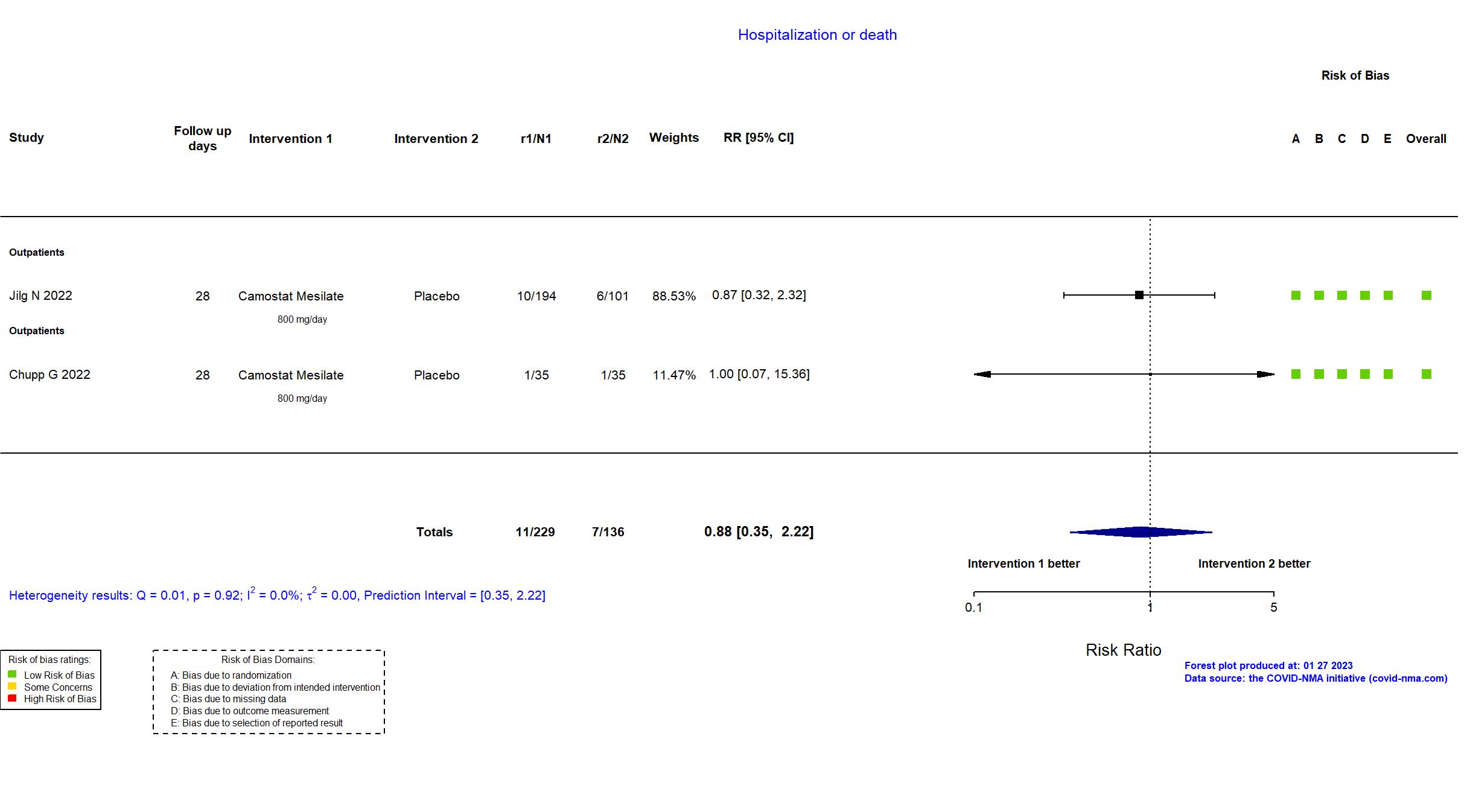
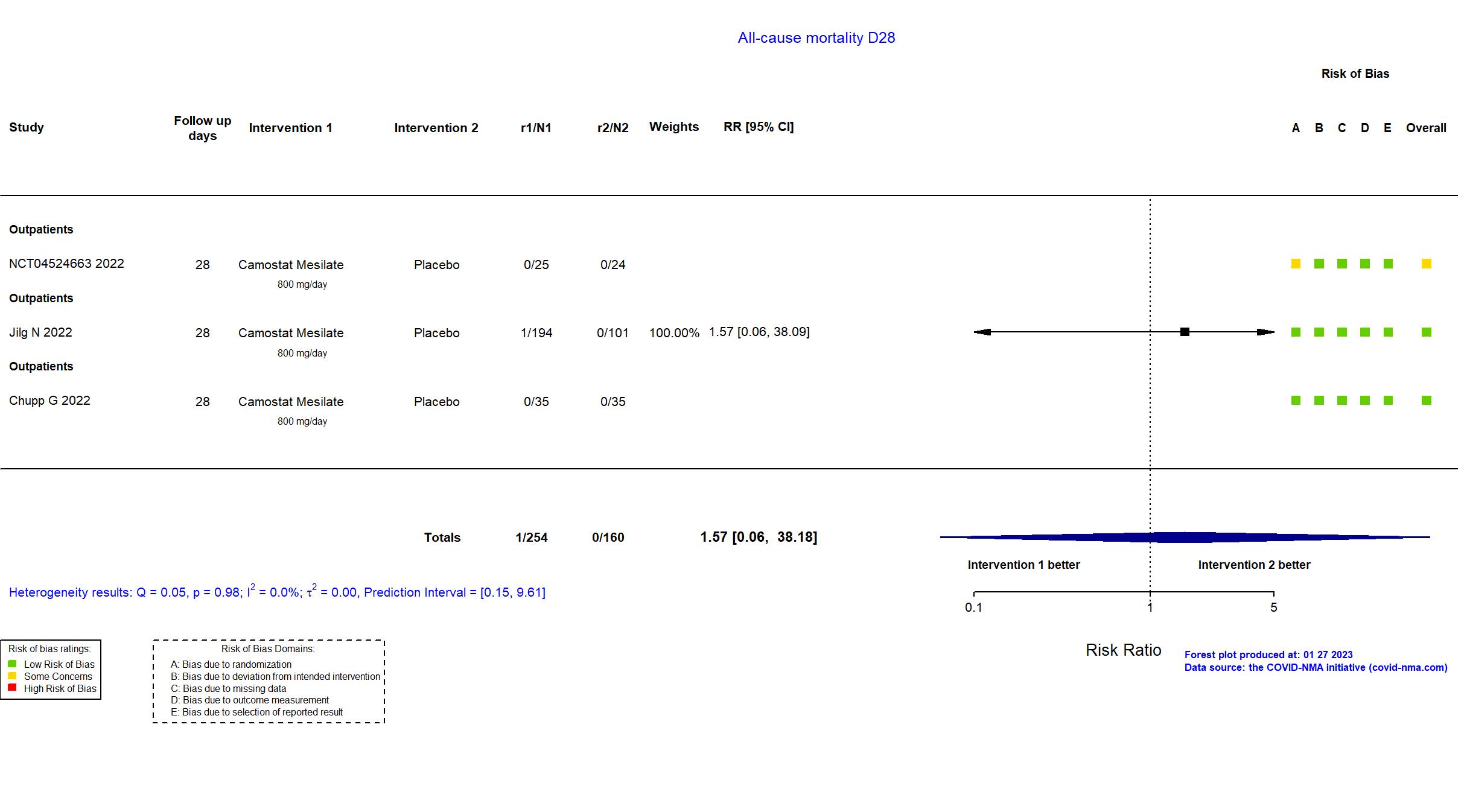
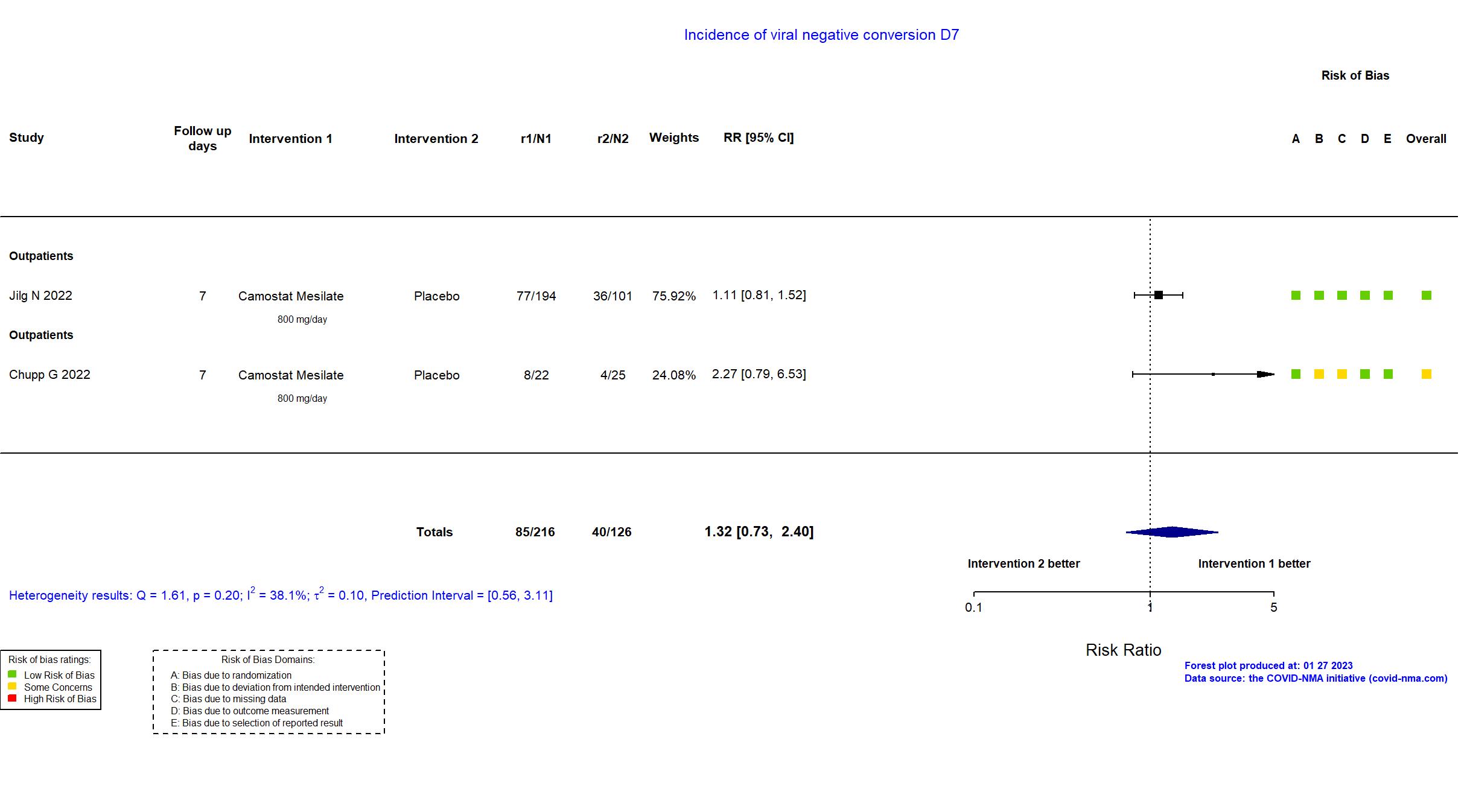
FOREST PLOTS -2023-01-27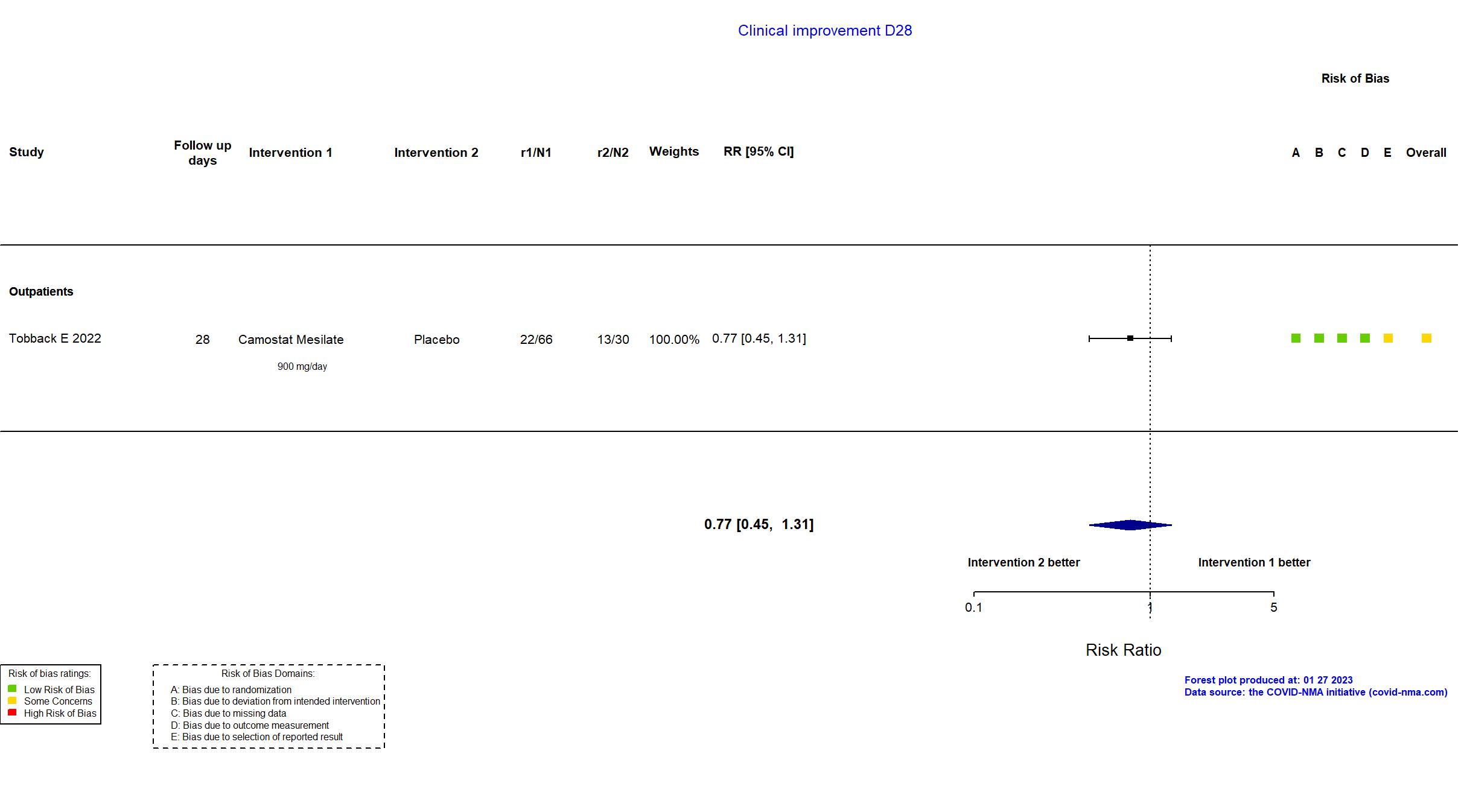
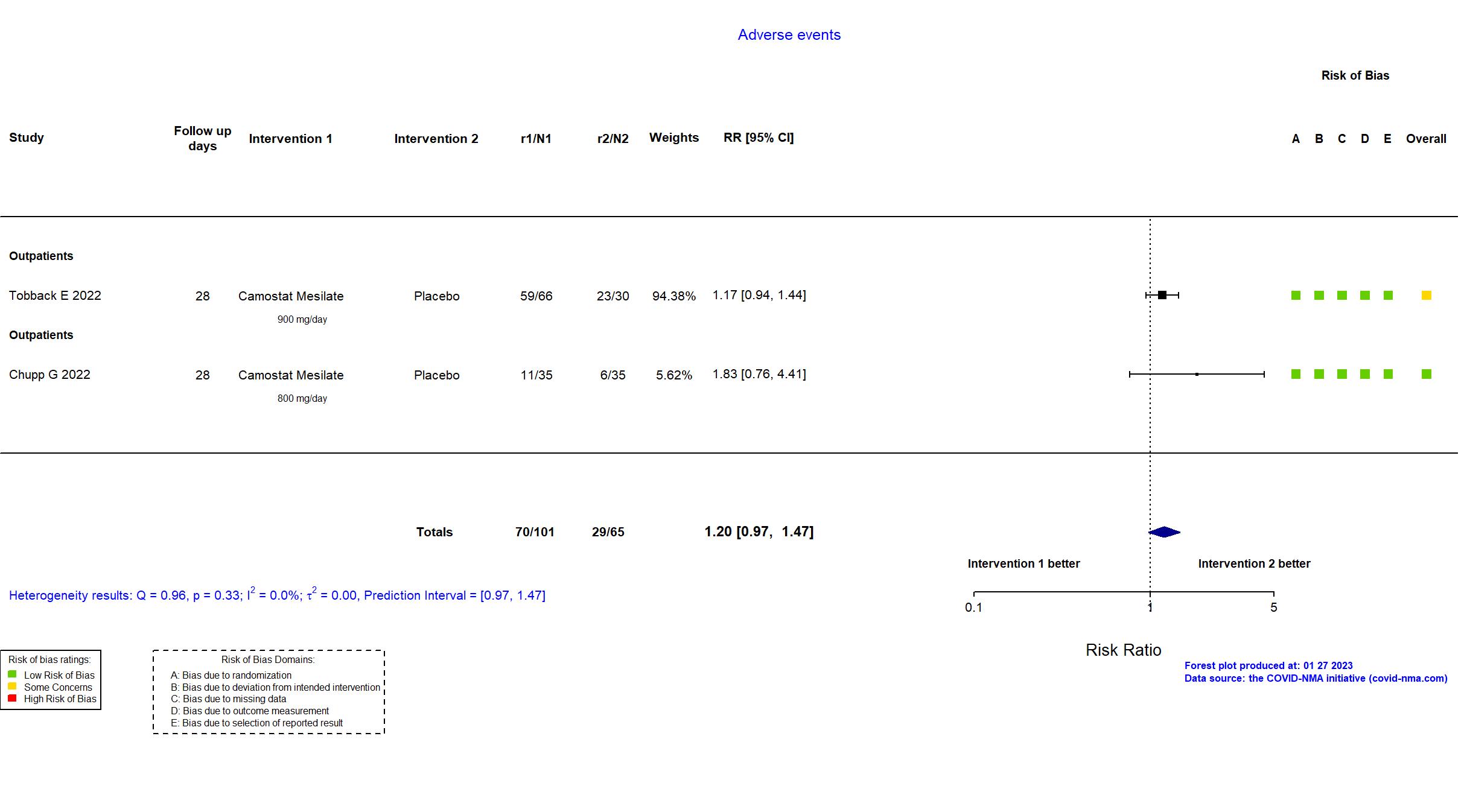
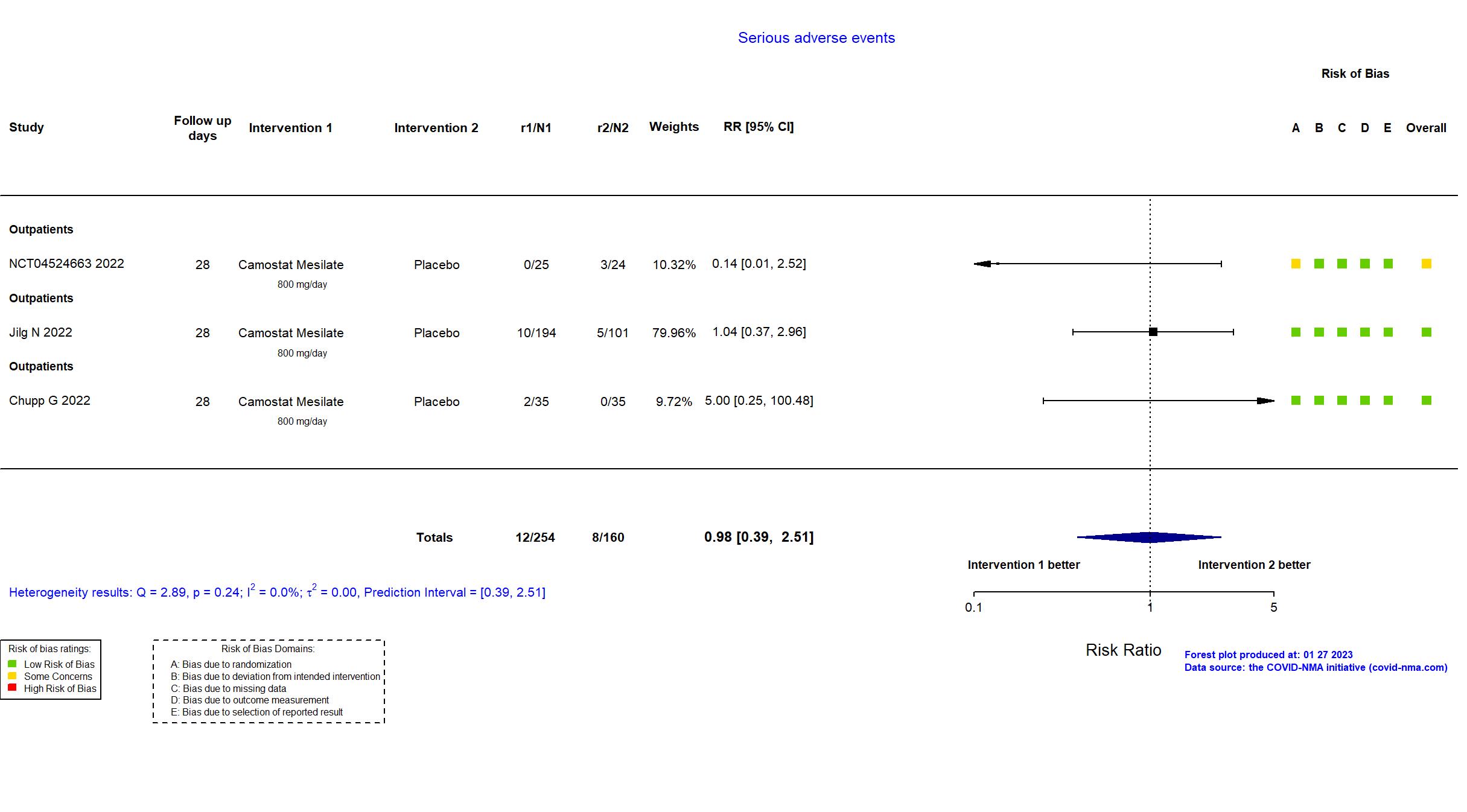
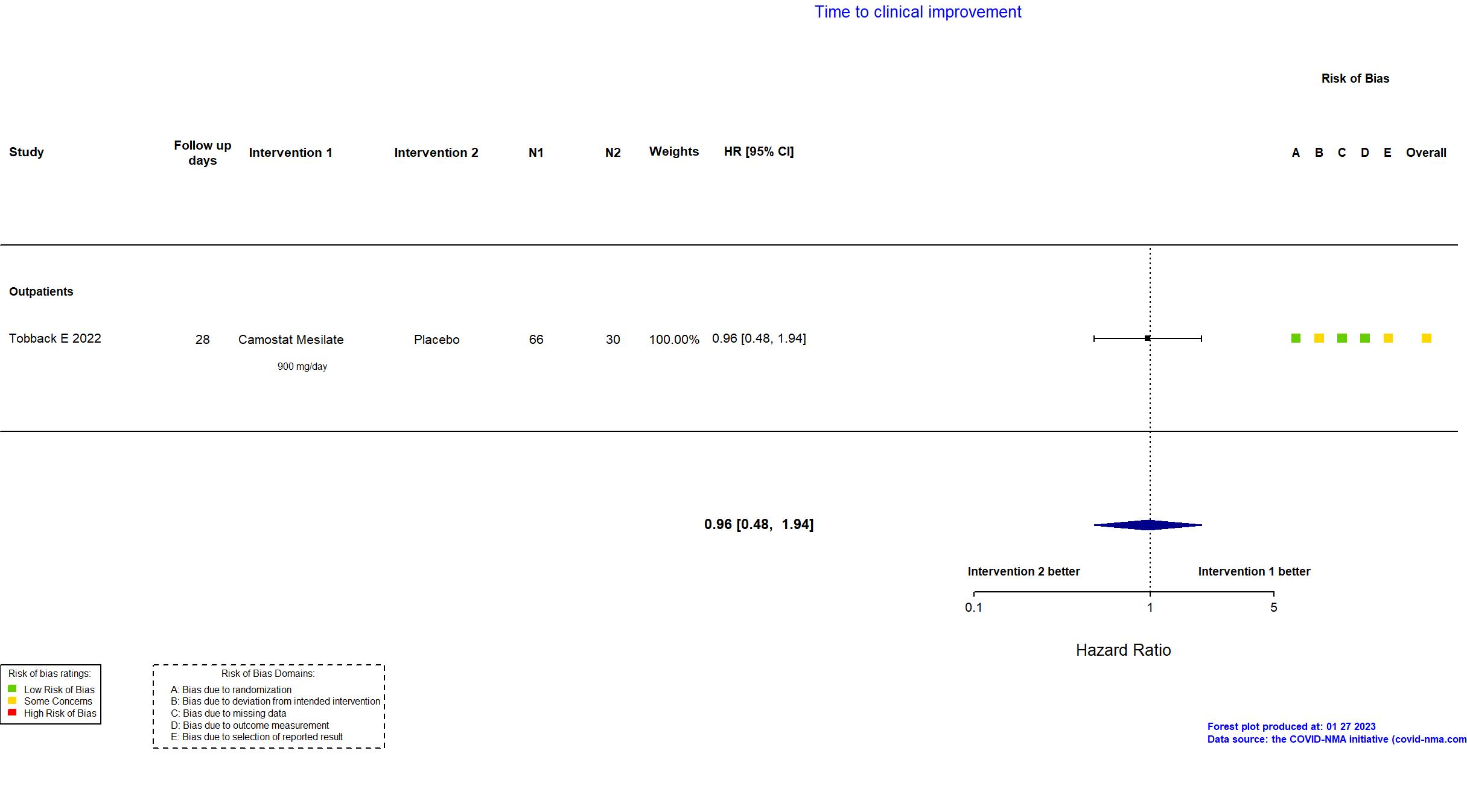
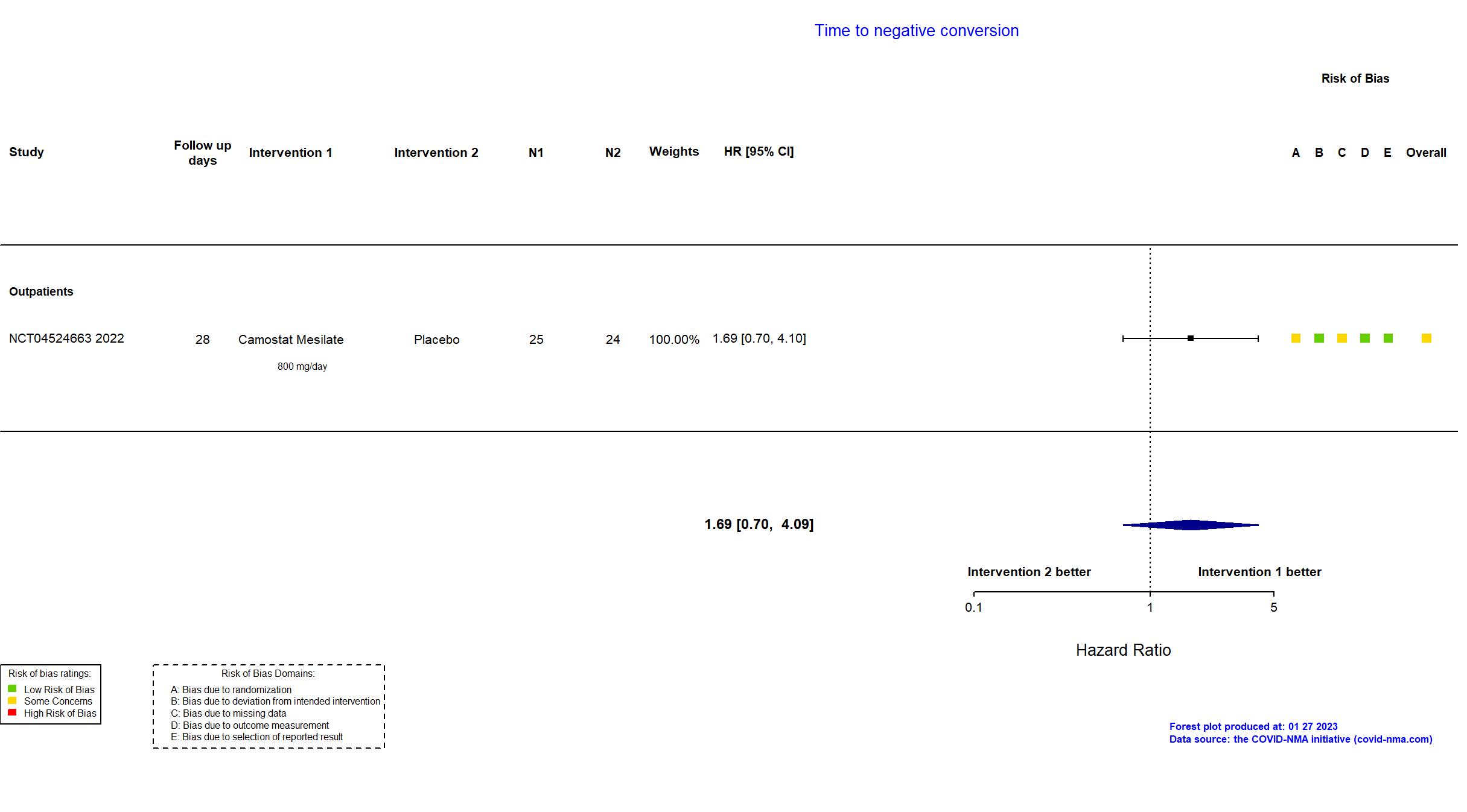








Trial NCT04353284
Publication Chupp G, medRxiv (2022) (preprint)
Dates: 2020-06-01 to 2021-04-30
Funding: Mixed (Kenneth C. Griffin; the Prostate Cancer Foundation; the COVID-19 Early Treatment Fund; the Harrington Discovery Institute; institutional funds from the Department of Internal Medicine at the Yale School of Medicine, and the Yale Center for Clinical Investigation; the United State Public Health Service; Ono Pharmaceuticals provided the study drug.)
Conflict of interest: No
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Single center / USA Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Camostat Mesilate 200 mg orally 4 times a day for 7 days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Camostat Mesilate=35 Placebo=35 | |
| Characteristics of participants N= 70 Mean age : NR 42 males Severity : Mild: n= 70/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register Change in SARS-COV-2 viral load [ Time Frame: 5 days ] To determine whether camostat mesylate reduces SARS-COV-2 viral load in early COVID-19 disease, change from day 0 to day 4 in respiratory (oropharyngeal swab RT-PCR) log10 viral load will be assessed. | |
| In the report Change in the log10 viral load of a NP swab specimen as determined by quantitative RT-PCR testing from baseline to day 4 post-randomization. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the pre-print article, the trial registry, protocol, statistical analysis plan and supplementary appendices were used in data extraction and assessment of risk of bias. The primary outcome in the article reflects that in the registry. The study (N = 70) did not achieve its target sample size (n = 114) because recruitment was terminated.
On October 20th, 2022, this study was updated with information extracted from registry. Of note, for the incidence of viral negative conversion outcome, results from both nasopharyngeal swab samples and saliva RT-PCR were reported; the latter was extracted. |
Trial NCT04518410
Publication ACTIV-2 - Jilg N, Top Antivir Med (2022) (unpublished results)
Funding: Not reported/unclear
Conflict of interest: *
| Methods | |
| RCT Blinding: triple blinding | |
| Location :
Multicenter / USA Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria | NR |
| Interventions | |
| Treatment
Camostat Mesilate 200 mg orally every 6 hours for 7 days |
|
| Control
Placebo | |
| Participants | |
| Randomized NR Analyzed 215 participants Placebo=107 Camostat Mesilate=108 | |
| Characteristics of participants N= 215 Mean age : NR 0 males Severity : Mild: n= */ Asymptomatic: n=* | |
| Primary outcome | |
| In the register 1) COVID-19 symptom duration (Phase 2) [ Time Frame: Up to Day 28 ] Duration defined as the number of days from start of investigational agent to the first of two consecutive days when any symptoms scored as moderate or severe as study entry (pre-treatment) are scored as mild or absent; and any symptoms scored as mild or absent at study entry are scored as absent, AND any symptoms scored as mile or absent at study entry (pre-treatment) are scored as absent. Targeted symptoms are: Feeling feverish; cough, shortness of breath or difficulty breathing; sore throat; body pain or muscle pain/aches; fatigue (low energy); headache, chills, nasal obstruction or congestion (stuffy nose); nasal discharge (runny nose); nausea or vomiting; and diarrhea. Each symptom is scored daily by the participant as absent (score 0), mild (1), moderate (2) or severe (3) 2) Quantification of SARS-CoV-2 RNA (Phase 2) [ Time Frame: Day 3 ] Measured as quantification ( | |
| In the report Safety and efficacy of camostat to reduce the duration of COVID-19 symptoms and increase the proportion of participants with SARS-CoV-2 RNA below the lower limit of quantification (LLoQ) from nasopharyngeal (NP) swabs on days 3, 7, and 14 | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
* |
| General comment | The published abstract and study registry were used in data extraction. No review specific outcomes were extractable. This study is part of the the ACTIV-2 platform trial. |
Trial NCT04583592
Publication CAMELOT - Jilg N, Top Antivir Med (2022) (results posted on registry)
Funding: Not reported/unclear
Conflict of interest: *
| Methods | |
| RCT Blinding: quadruple blinding | |
| Location :
Multicenter / USA Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Camostat Mesilate 200 mg orally 4 times a day for 14 days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Placebo=101 Camostat Mesilate=194 | |
| Characteristics of participants N= 295 Mean age : NR 126 males Severity : Mild: n= 295/ Asymptomatic: n=* | |
| Primary outcome | |
| In the register Disease Progression at Day 28 [ Time Frame: 28 days ] Disease progression will be defined as the number of participants requiring hospitalization (including emergency room visit) or who die due to any cause within 28 days of randomization. | |
| In the report Hospitalization or death within 28 days | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Low |
| General comment |
This is an unpublished trial whose results have been reported in ClinicalTrials.gov. The trial registry, protocol and statistical analysis plan were used in data extraction and assessment of risk of bias. The trial was registered prospectively and no important changes were made to primary or secondary outcomes after recruitment start. The trial (n=295) achieved its target sample size (n=300).
This study was updated on January 26th, 2023 with data extracted from the published abstract. |
Trial NCT04524663
Publication COPS-2003 - NCT04524663, Unpublished (2022) (results posted on registry)
Funding: Not reported/unclear
Conflict of interest: *
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Single center / USA Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Camostat Mesilate 200 mg orally 4 times per day for 10 days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Camostat Mesilate=25 Placebo=24 | |
| Characteristics of participants N= 49 Mean age : NR 32 males Severity : Mild: n= 49/ Asymptomatic: n=* | |
| Primary outcome | |
| In the register Area Under the Curve (AUC) of Shedding of SARS-CoV-2 Virus [ Time Frame: Days 1-10 ] | |
| In the report NR | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | This is an unpublished trial whose results have been reported in ClinicalTrials.gov. The trial registry, protocol and statistical analysis plan were used in data extraction and assessment of risk of bias. The trial was registered prospectively and no important changes were made to primary or secondary outcomes after recruitment start. |
Trial NCT04625114
Publication Tobback E, Int J Infect Dis (2022) (published paper)
Dates: 2020-11-04 to 2021-06-30
Funding: Mixed (Ono Pharmaceuticals Co. Ltd. (Osaka, Japan) provided the study drug, camostat mesylate. Byteflies (Antwerp, Belgium) provided telemonitoring devices and technological support. No further funding was received.)
Conflict of interest: No
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Single center / Belgium Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Camostat Mesilate 300 mg orally three times a day for 5 days, with possible extension to 10 days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Camostat Mesilate=66 Placebo=30 | |
| Characteristics of participants N= 96 Mean age : NR 41 males Severity : Mild: n= 77/ Asymptomatic: n=13 | |
| Primary outcome | |
| In the register Efficacy in terms of viral load or surrogate [ Time Frame: 5 days ] The primary endpoint is to assess the efficacy of the drug in terms of change from day 0 to day 5 in respiratory (oropharyngeal swab RT-PCR) log10 viral load. Surrogate market CT value will be used as well. | |
| In the report Change in the shedding of the SARS-CoV-2 virus as measured by Ct obtained from nasopharyngeal swabs on days 1 and 5. | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the trial registry was used in data extraction and assessment of risk of bias. Neither protocol nor statistical analysis plan was available. The primary outcome in the article reflects that in the registry. The trial did not achieve its target sample size due to a lack of available enrollees and the per protocol decision was taken to conduct this interim analysis, after which recruitment was stopped. |