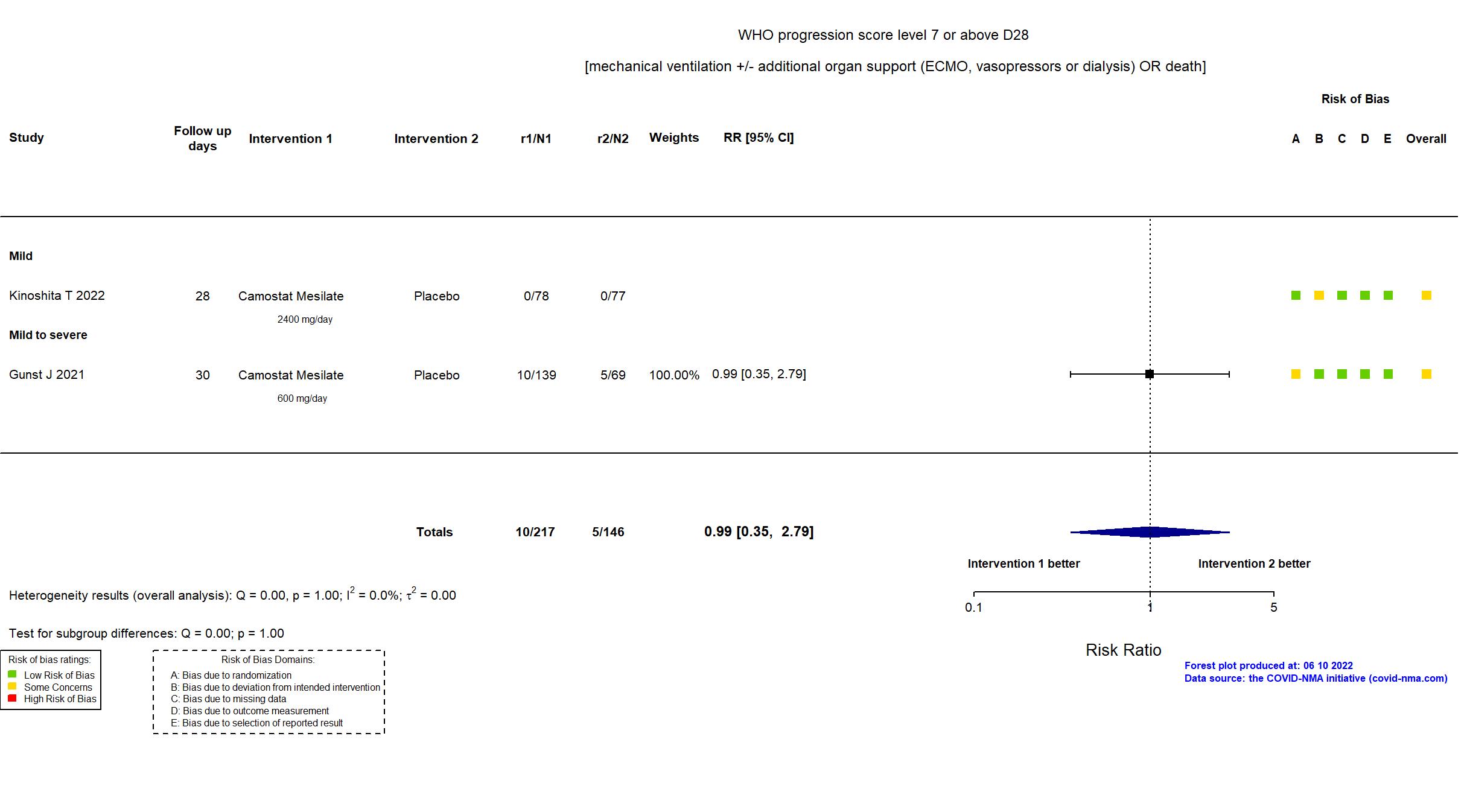
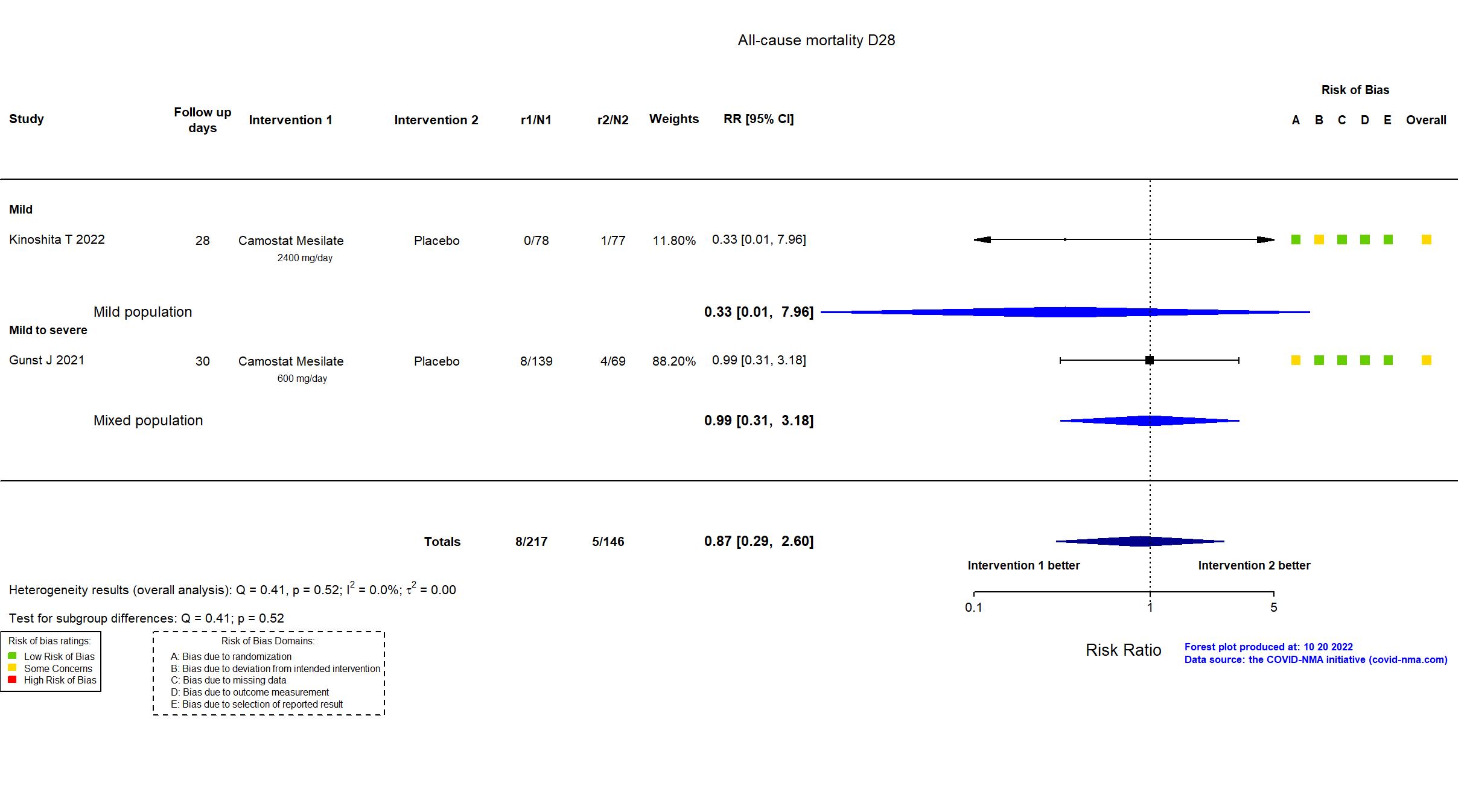
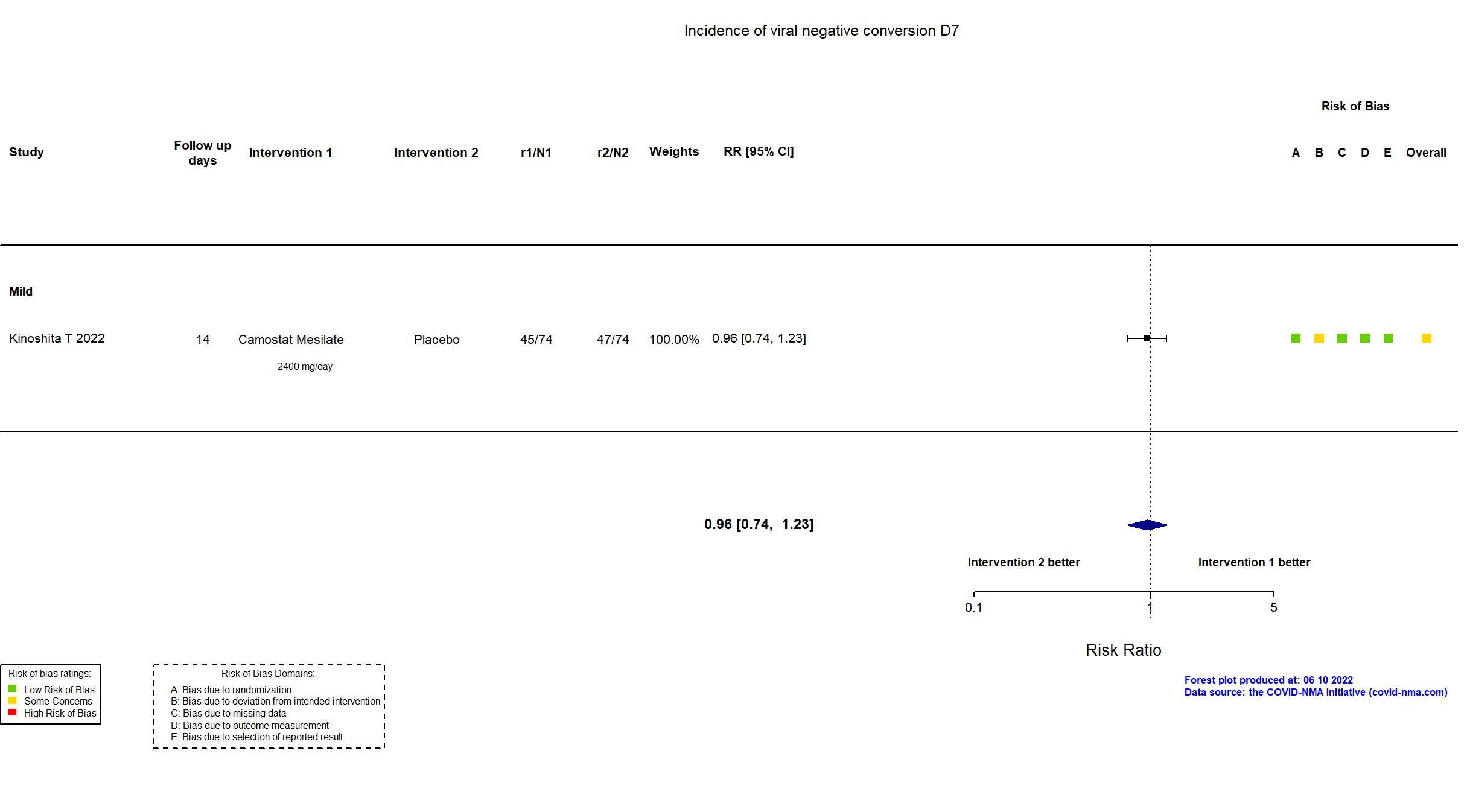
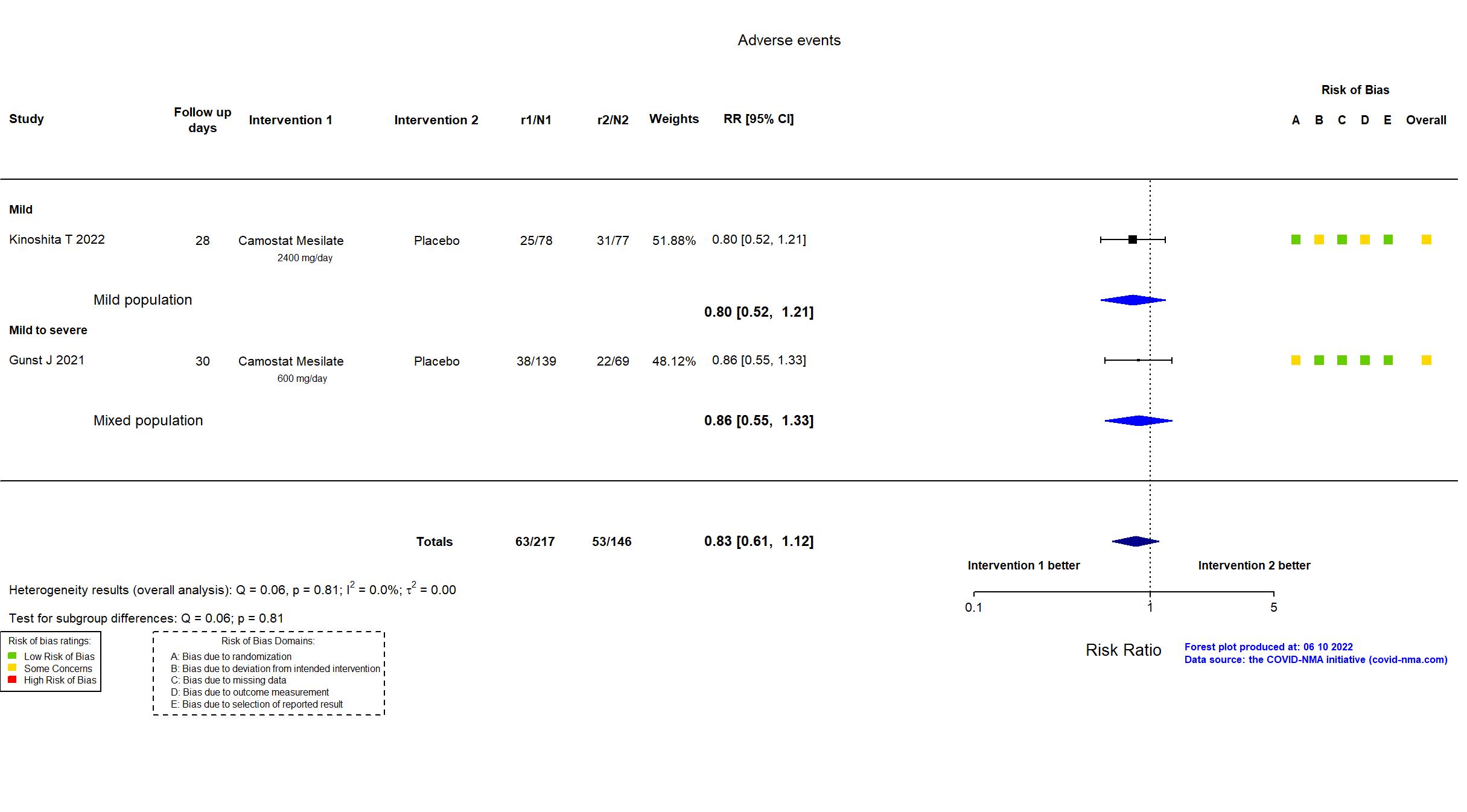
Camostat Mesilate vs Placebo (RCT)
Hospitalized patients
FOREST PLOTS -2022-10-20
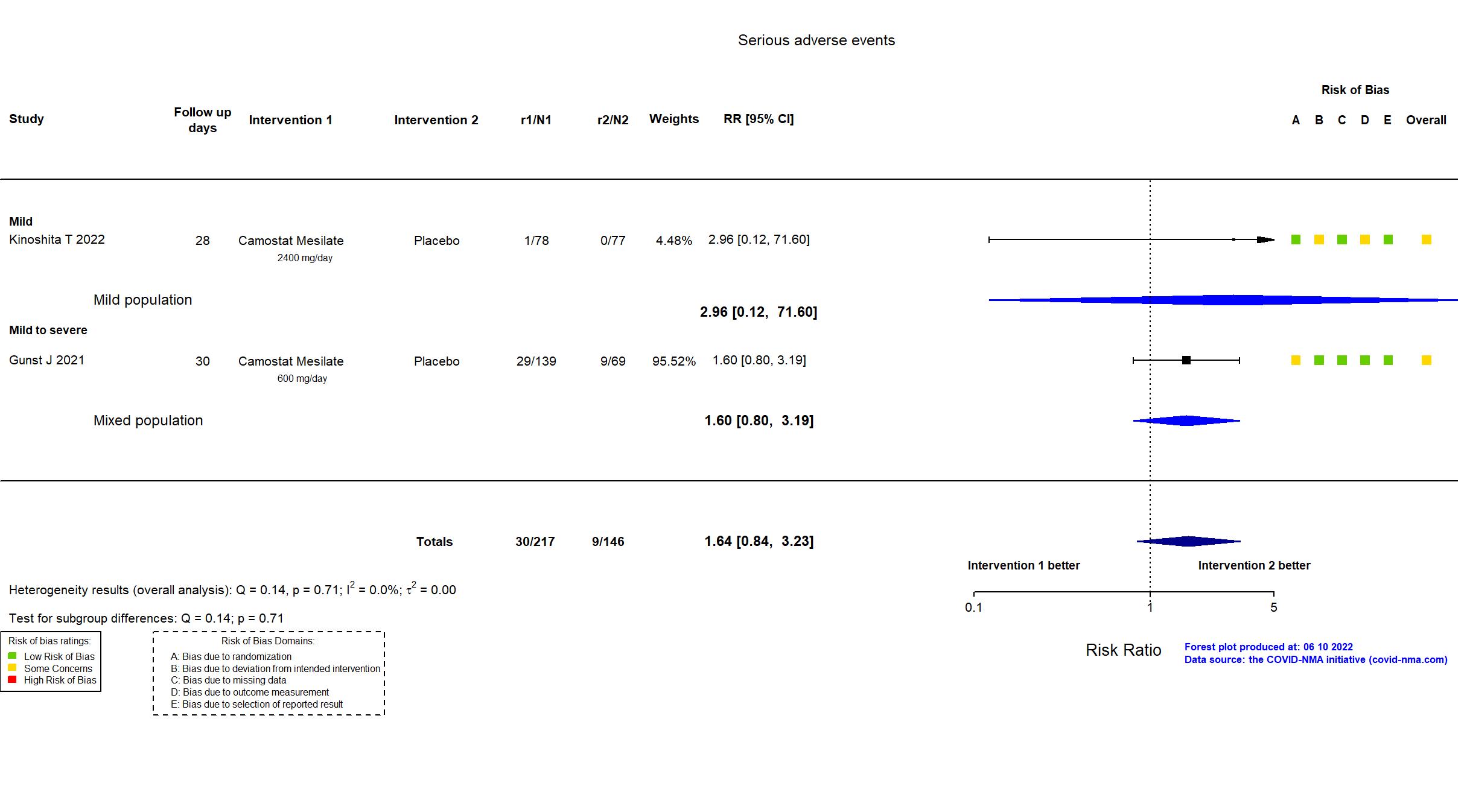

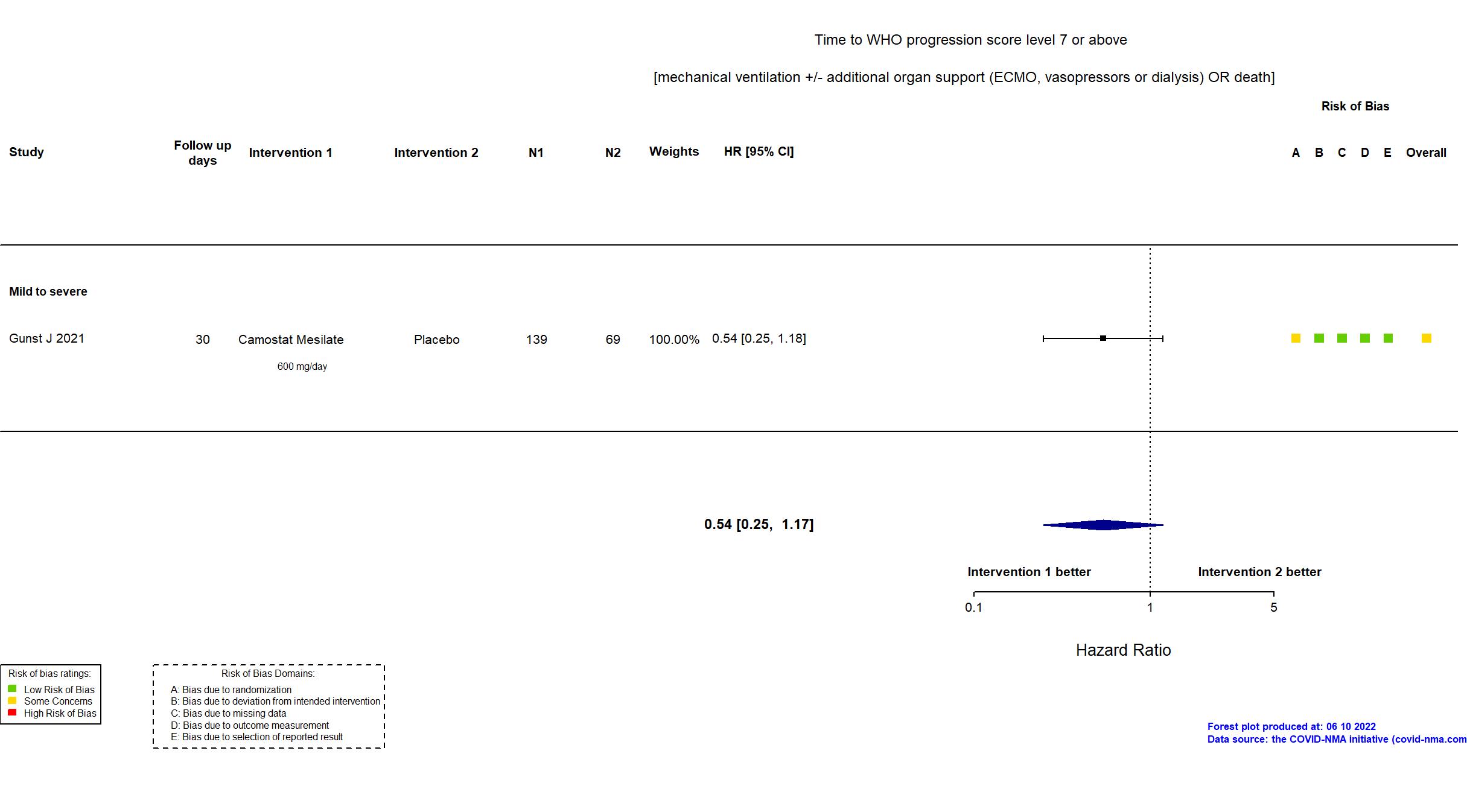
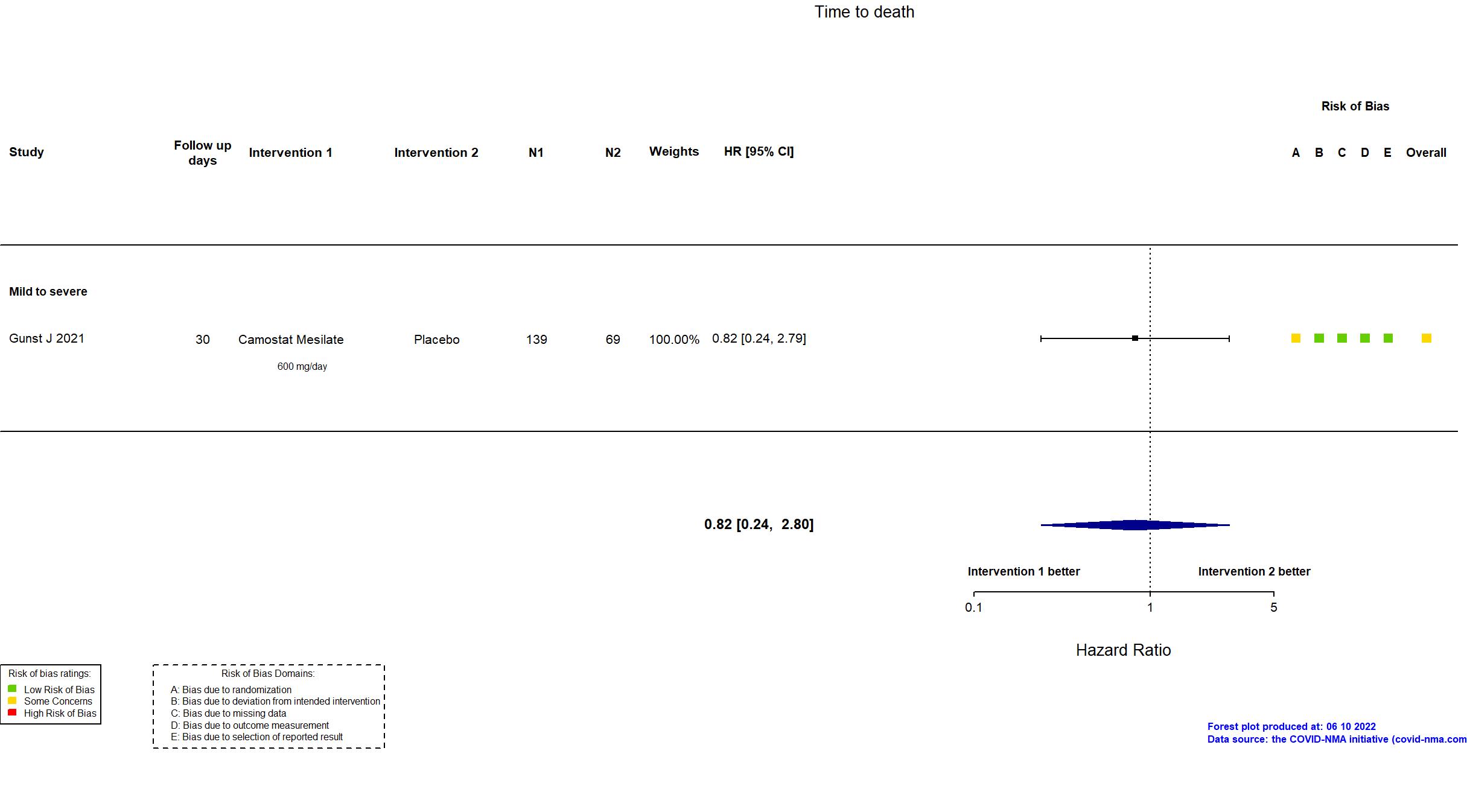
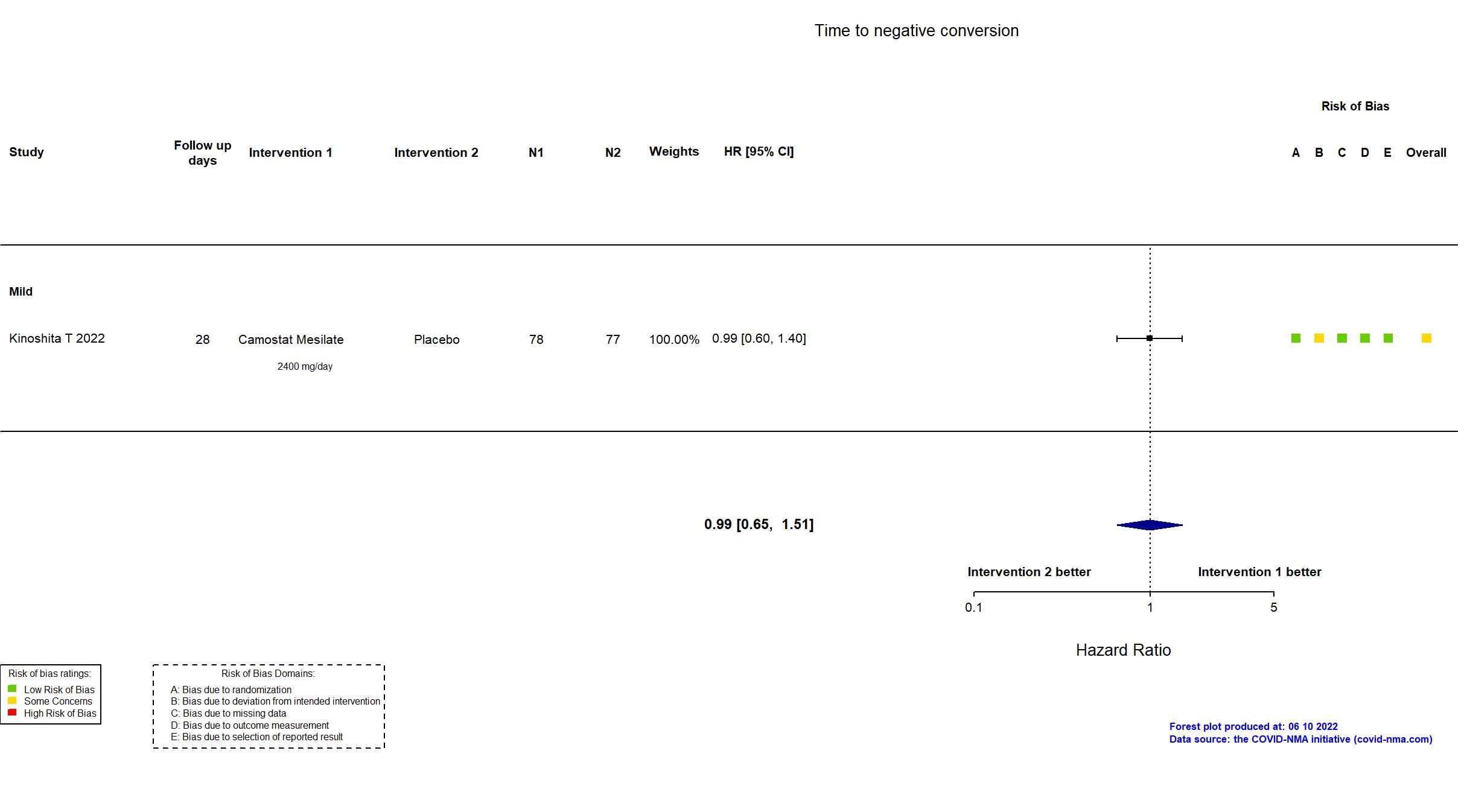










Trial NCT04321096 ; EudraCT Number: 2020001,20042
Publication Gunst J, EClinicalMedicine (2021) (published paper)
Dates: 2020-04-04 to 2020-12-31
Funding: Private (The Lundbeck Foundation)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: | |
| Location :
Multicenter / Denmark, Sweden Follow-up duration (days): 30 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Camostat Mesilate 200 mg three times a day for five days. |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Camostat Mesilate=139 Placebo=69 | |
| Characteristics of participants N= 208 Mean age : NR 123 males Severity : Mild: n=69 / Moderate: n=120 / Severe: n=16 Critical: n=0 | |
| Primary outcome | |
| In the register Clinical improvement defined as live hospital discharge OR a 2 point improvement (from time of enrolment) in disease severity rating on the 7-point ordinal scale. | |
| In the report Time to clinical improvement, defined as live hospital discharge or an improvement of at least 2 points from baseline on the 7-point ordinal scale, which ever came first | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the trial registry, protocol, statistical analysis plan (in the protocol) and supplementary files were used in data extraction and assessment of risk of bias. There were no substantive differences in population, procedures, or interventions between the published article and the trial registry, protocol and statistical analysis plan. The outcome 'Days in ICU' was specified in the trial protocol but not in the trial registry nor in the published paper. The article reports the inpatient part of a two-part trial that has two cohorts: one inpatients, one outpatients. The study achieved its pre-specified target sample size. |
Trial NCT04657497; jRCT2031200198
Publication CANDLE - Kinoshita T, BMC Medicine (2022) (published paper)
Dates: 2020-11-09 to 2021-03-30
Funding: Private (Ono Pharmaceutical Co., Ltd)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / Japan Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Camostat Mesilate 600 mg orally four times daily for up to 14 days. |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Camostat Mesilate=78 Placebo=77 | |
| Characteristics of participants N= 155 Mean age : NR 78 males Severity : Mild: n=108 / Moderate: n=0 / Severe: n=0 Critical: n=0 | |
| Primary outcome | |
| In the register Time to SARS-CoV-2 negative test [ Time Frame: Up to 14 days ] | |
| In the report Time to the first two consecutive negative SARS-CoV-2 tests | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the pre-print article, the prospective protocol, statistical analysis plan, supplementary appendix and prospective study registry were used in data extraction and risk of bias assessment. There is no change from the trial registration in the intervention and control treatments. The primary and secondary outcomes indicated in protocol and registry reflect the outcomes reported in the paper. The study (n=155) achieved the target sample size (n=110) specified in the protocol.
On October 20tg, 2020, this study was updated with information extracted from the publication. |