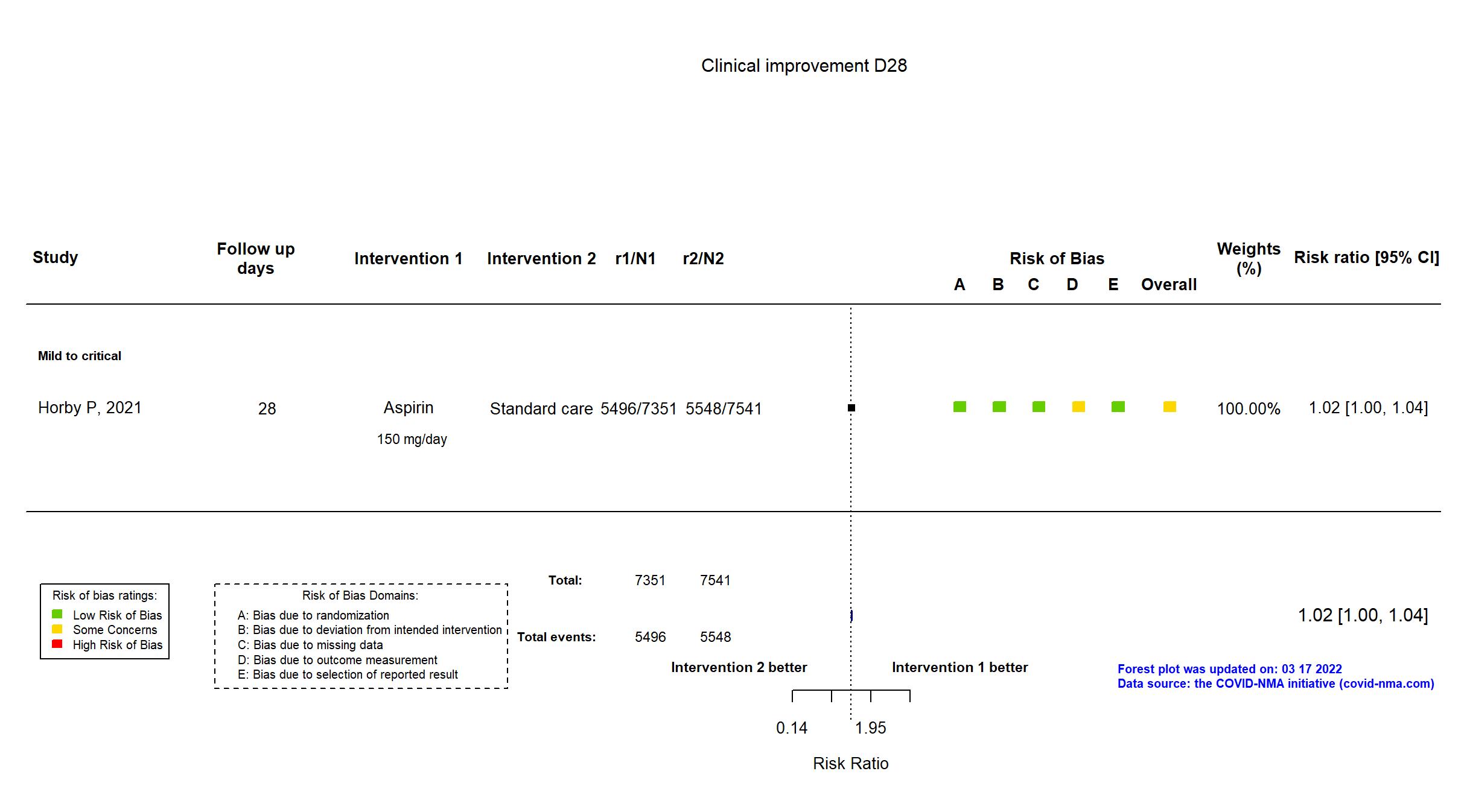
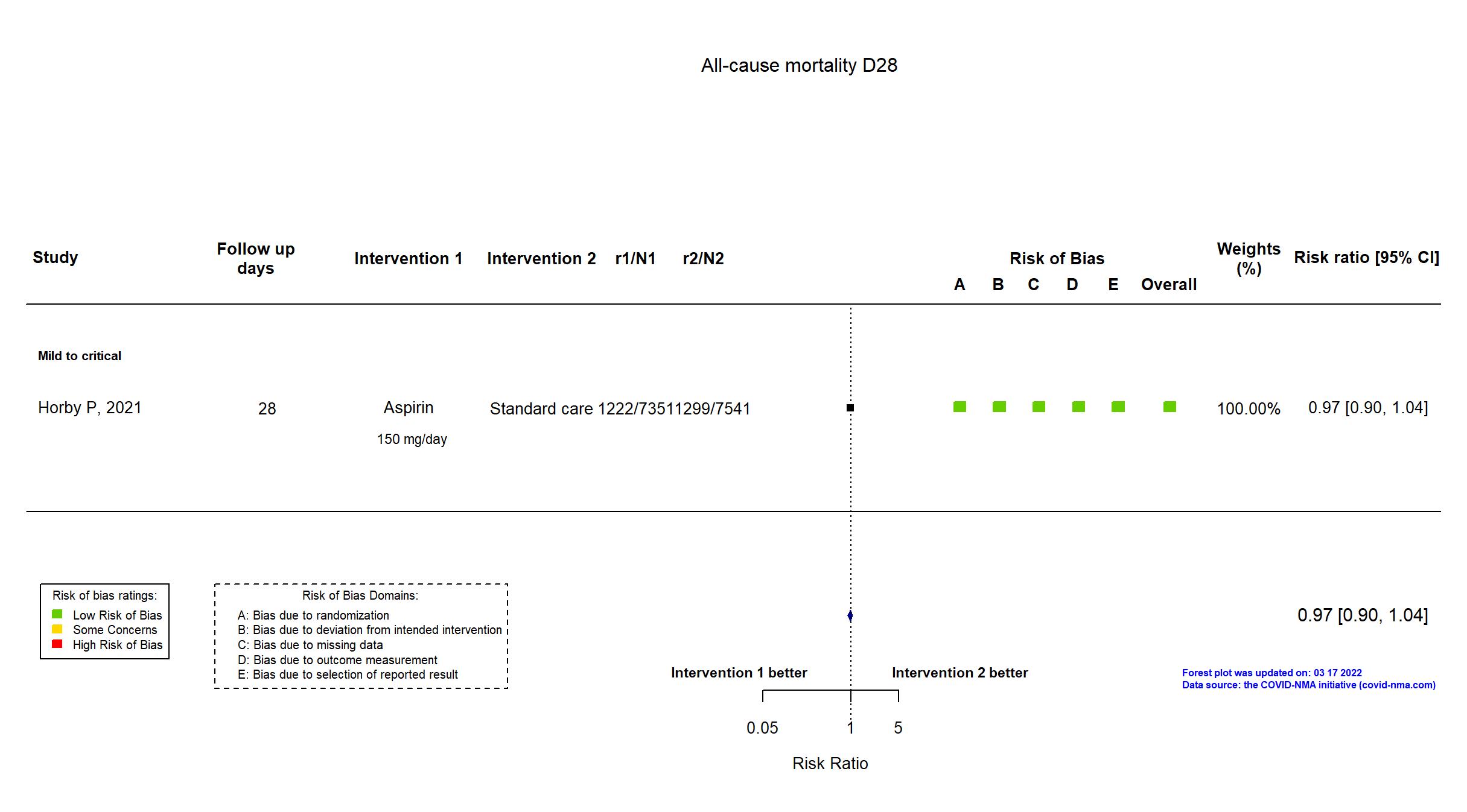
Aspirin vs Standard care/Placebo (RCT)
Hospitalized patients
On March 17, 2022 study Ghati, 2021 was excluded from the analysis since the paper is not anymore accessible on SSRN nor on published elsewhere. We have contacted authors for further details. FOREST PLOTS -2022-03-17