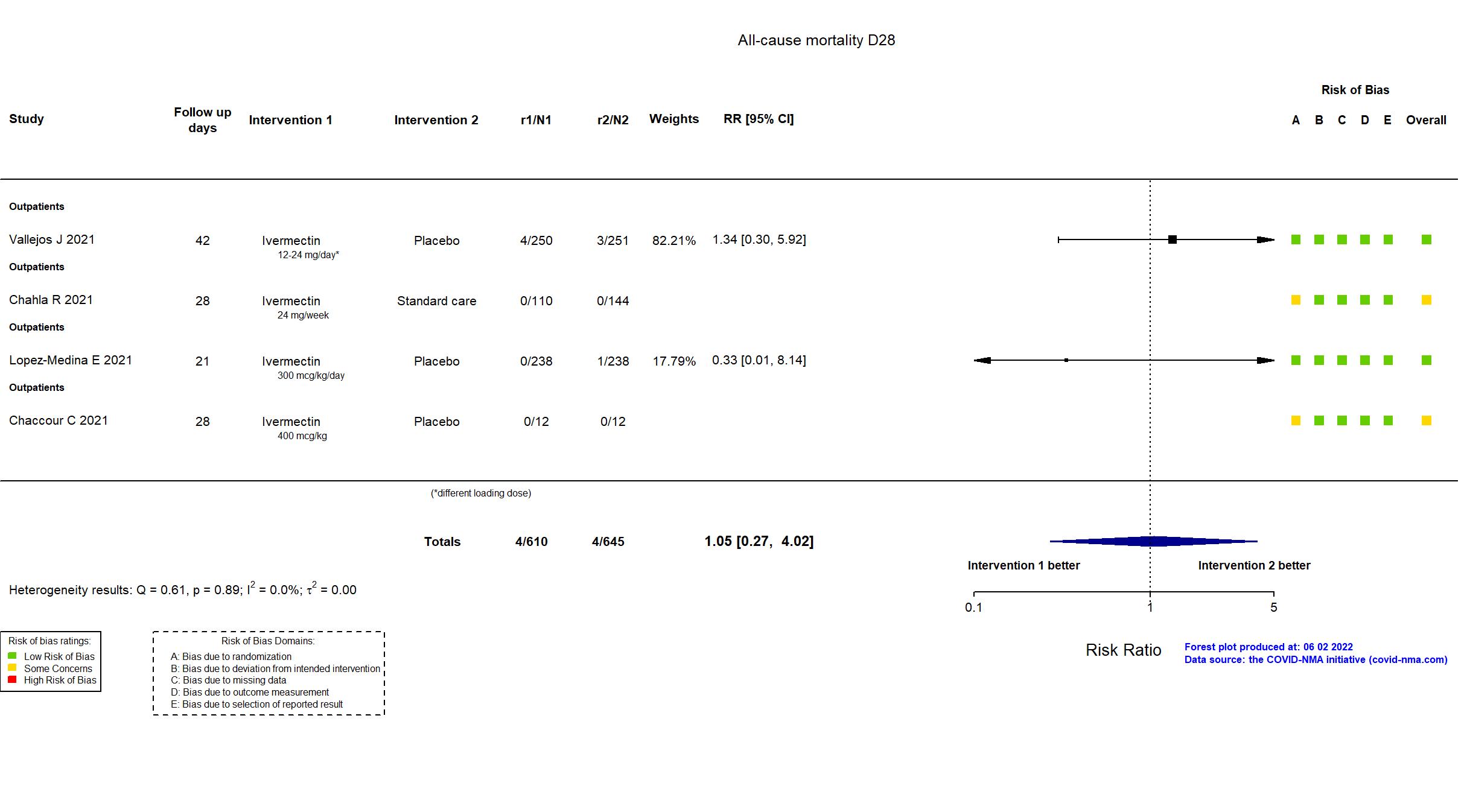
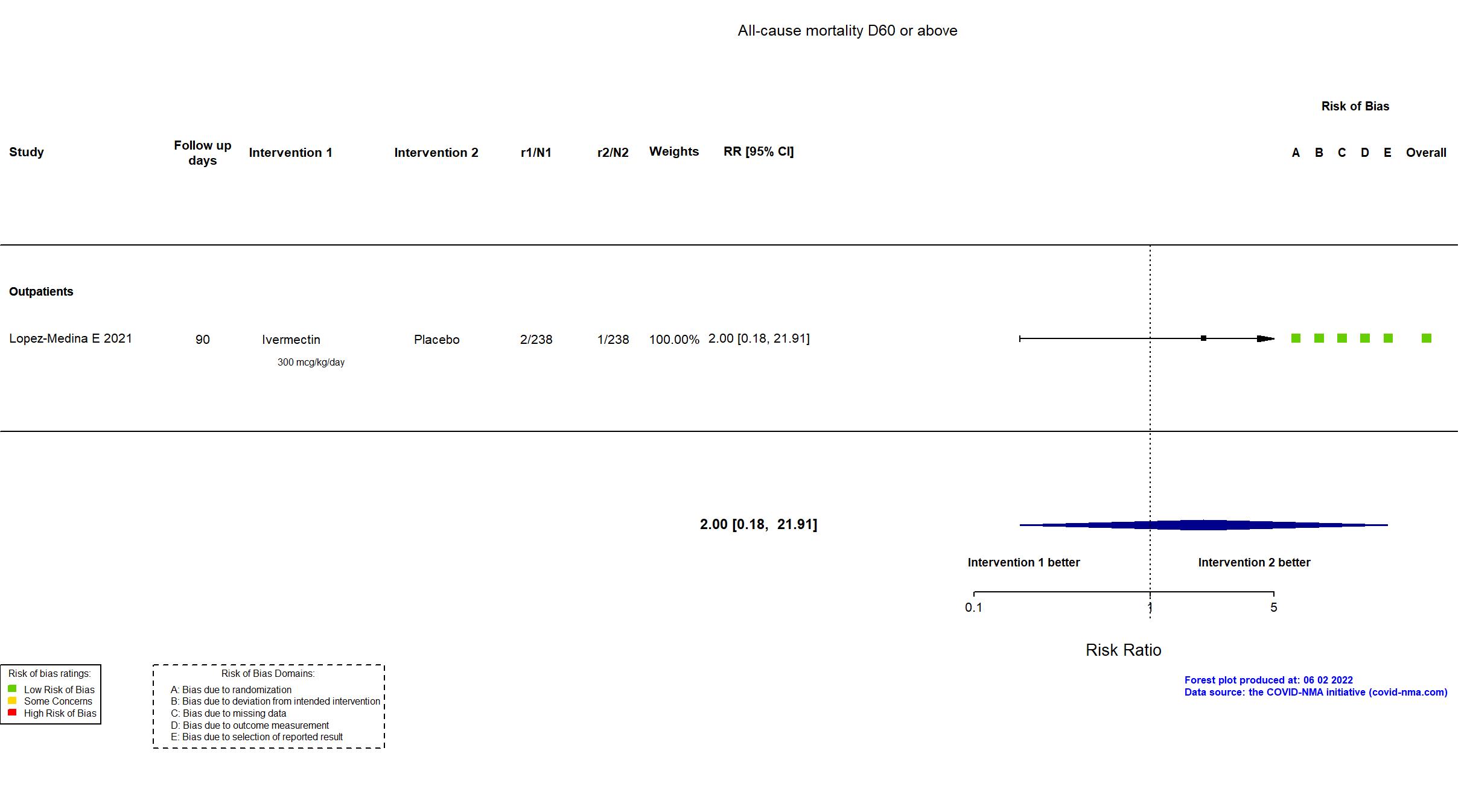
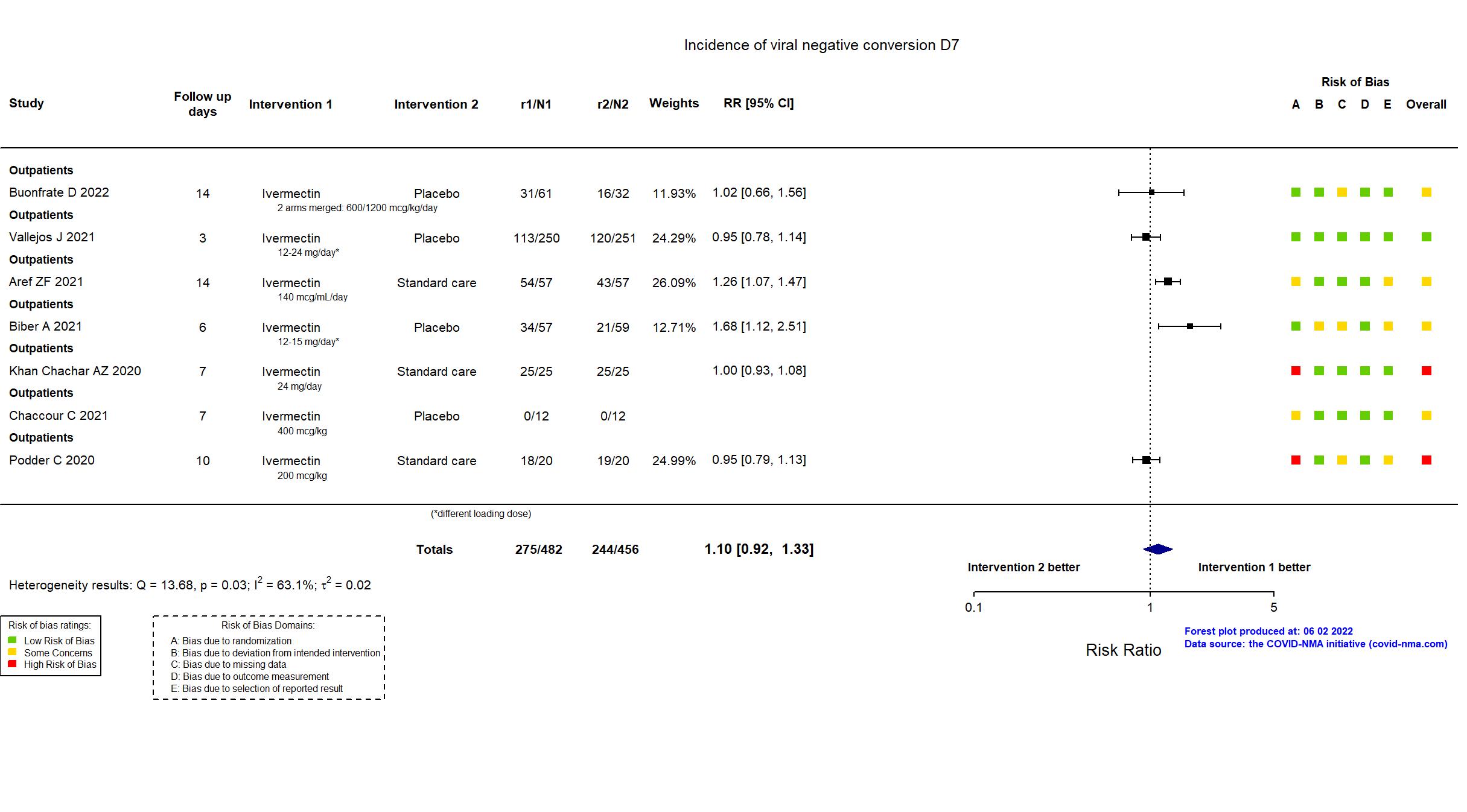
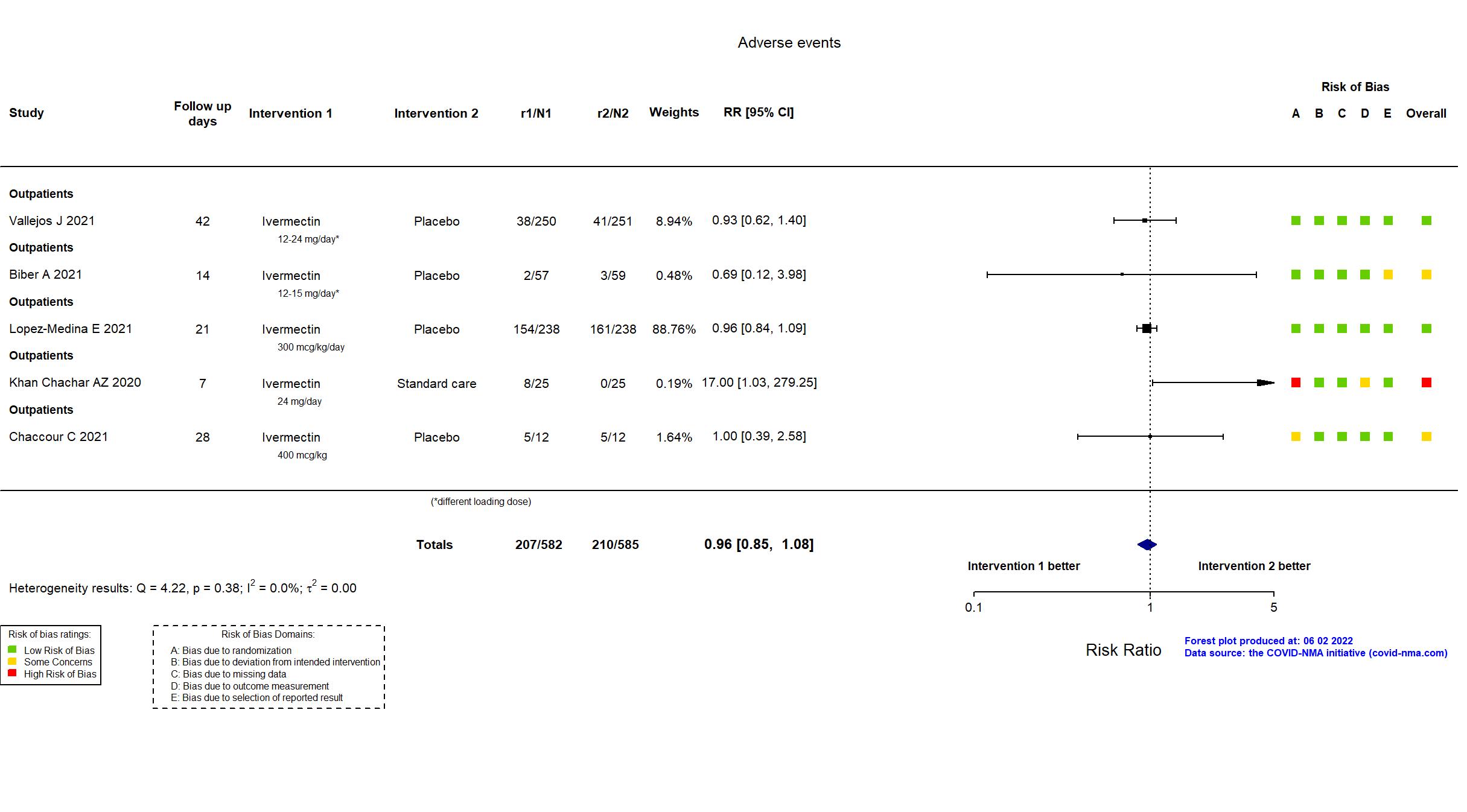
Studies description
Trial NCT04716569
Publication Aref ZF, Int J Nanomed (2021) (published paper)
Dates: 2021-02-20 to 2021-03-30
Funding: Public/non profit (South Valley University, Faculty of Medicine)
Conflict of interest: No
Trial NCT04429711
Publication Biber A, medRxiv (2021) (preprint)
Dates: 2020-05-15 to 2021-01-30
Funding: No specific funding (None)
Conflict of interest: No
Trial NCT04438850
Publication COVER - Buonfrate D, Int J Antimicrob Agents (2022) (published paper)
Dates: 2020-07-31 to 2021-06-10
Funding: Public/non profit (Italian Ministry of Health; Tablets of 9 mg ivermectin and of placebo were donated by Insud Pharma, Madrid.)
Conflict of interest: No
Trial NCT04390022
Publication SAINT - Chaccour C, EClinicalMedicine (2021) (published paper)
Dates: 2020-07-31 to 2020-09-11
Funding: Mixed (ISGlobal; University of Navarra. Unitaid; Spanish Ministry of Science and Innovation; Generalitat de Catalunya; Idipharma SL (placebo donation))
Conflict of interest: No
Trial NCT04784481
Publication Chahla R, Research Square (2021) (preprint)
Funding: Public/non profit (Ministry of Public Health, Tucumán, Argentina)
Conflict of interest: No
Trial NCT04739410
Publication Khan Chachar AZ, Int J Sci (2020) (published paper)
Funding: No specific funding
Conflict of interest: *
Trial NCT04405843
Publication Lopez-Medina E, JAMA (2021) (published paper)
Dates: 2020-07-15 to 2020-11-30
Funding: Mixed (Centro de Estudios en Infectologia Pediatrica; Tecnoquimicas (drug and placebo donation))
Conflict of interest: Yes
Trial *
Publication Podder C, IMC J Med Sci (2020) (published paper)
Dates: 2020-05-01 to 2020-07-31
Funding: No specific funding (Self-financed)
Conflict of interest: No
Trial NCT04529525
Publication IVERCOR-COVID19 - Vallejos J, BMC Infect Dis (2021) (published paper)
Dates: 2020-08-19 to 2021-02-22
Funding: No specific funding (None)
Conflict of interest: No