Studies description
Trial NCT04403555
Publication Abd-Elsalam S, J Med Virol (2021) (published paper)
Dates: 2020-03-01 to 2020-10-30
Funding: Not reported/unclear
Conflict of interest: No
Trial NCT04407130
Publication Ahmed S, Int J Infect Dis (2020) (published paper)
Funding: Private (Beximco Pharmaceutical Limited, Bangladesh)
Conflict of interest: No
Trial NCT04391127
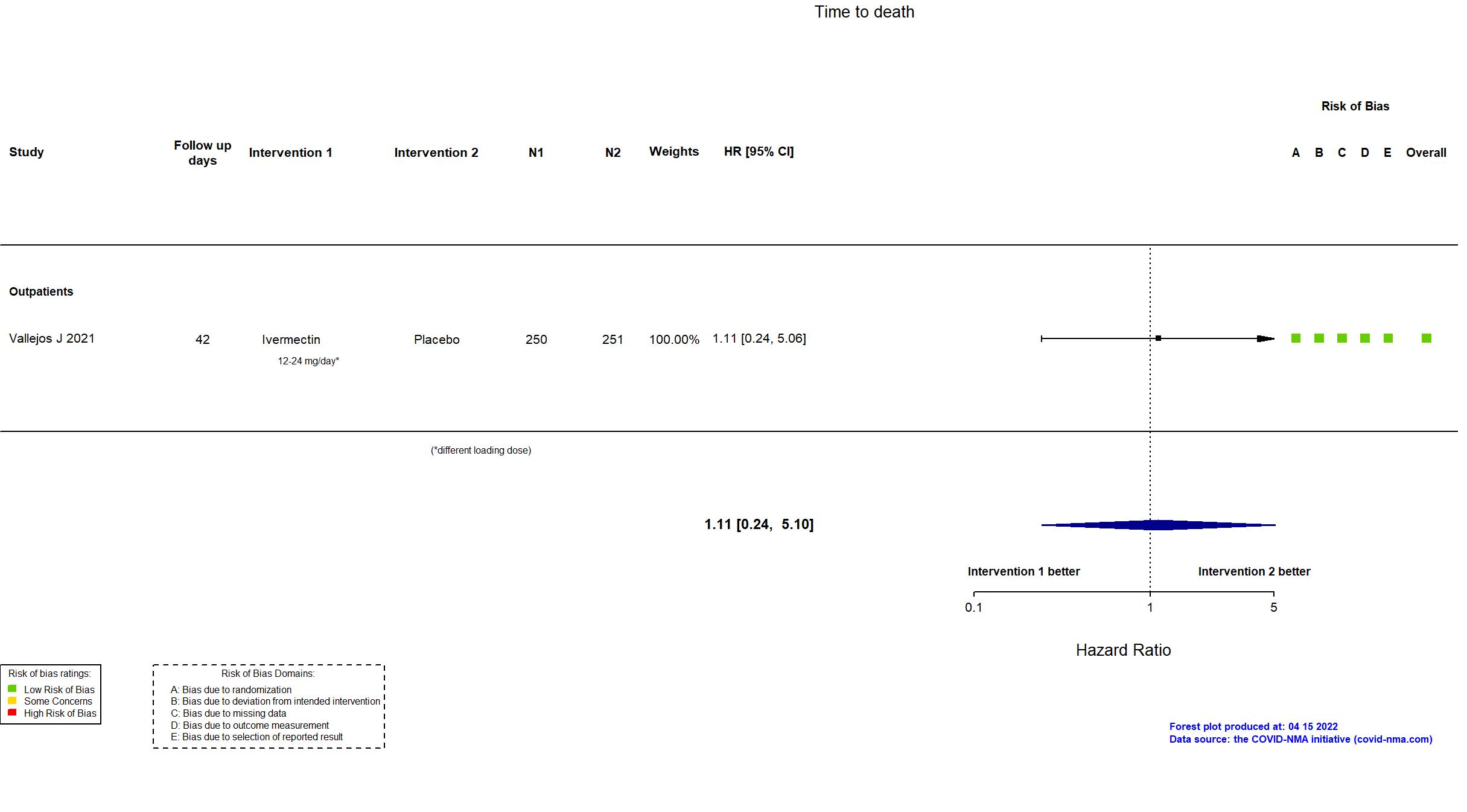
Publication Beltran-Gonzalez J, medRxiv (2021) (preprint)
Dates: 2020-05-04 to 2020-08-15
Funding: Public/non profit (Aguascalienes State Health Institute)
Conflict of interest: No
Trial *
Publication Kishoria N, PIJR (2020) (published paper)
Funding: Not reported/unclear
Conflict of interest: *
Trial NCT04381884
Publication Krolewiecki A, EClinicalMedicine (2021) (published paper)
Dates: 2020-05-18 to 2020-09-09
Funding: Mixed (Agencia Nacional de Promocion de la Investigacion, el Desarrollo Tecnologico y la Innovacion, Argentina and Laboratorio ELEA/Phoenix, Argentina)
Conflict of interest: Yes
Trial NCT04920942
Publication I-TECH - Lim SCL, JAMA Intern Med (2022) (published paper)
Dates: 2021-05-31 to 2021-10-09
Funding: Not reported/unclear
Conflict of interest: No
Trial *
Publication Manomaipiboon A, Research square (2022) (preprint)
Dates: 2021-09-01 to 2021-11-30
Funding: Public/non profit (Navamindradhiraj University)
Conflict of interest: No
Trial CTRI/2020/06/026001
Publication RIVET-COV - Mohan A, J Infect Chemother (2021) (published paper)
Dates: 2020-07-28 to 2020-09-29
Funding: Mixed (Department of Science and Technology, Government of India; WindLas BioTech Ltd. Haryana (drug contribution))
Conflict of interest: No
Trial IRCT20200408046987N1
Publication Niaee MS, Asian Pac J Trop Med (2021) (published paper)
Dates: 2020-06-01 to 2020-07-15
Funding: Mixed (Qazvin University of Medical Sciences and Science (publication); Chistasazan Notash Fartak Company Limited (registry))
Conflict of interest: *
Trial NCT04646109
Publication Okumus N, BMC Infectious Disea (2021) (published paper)
Dates: 2020-05-11 to 2020-09-02
Funding: Public/non profit (Afyonkarahisar Health Science University)
Conflict of interest: No
Trial CTRI/2020/08/027225
Publication Ravikirti R, J Pharm Pharm Sci (2021) (published paper)
Dates: 2020-08-28 to 2020-10-31
Funding: Mixed (All India Institute of Medical Sciences; Sun Pharma Pvt. Ltd. (placebo provision); learning resource allowance of the principal investigator (ivermectin procurement))
Conflict of interest: No
Trial NCT04392713
Publication Shah Bukhari K H, medRxiv (2021) (preprint)
Funding: Not reported/unclear ("Not applicable")
Conflict of interest: No
Trial IRCT20111224008507N3
Publication Shahbaznejad L, Clin Ther (2021) (published paper)
Dates: 2020-05-23 to 2020-07-31
Funding: No specific funding (Mazandaran University of Medical Sciences)
Conflict of interest: No