Otilimab vs Placebo (RCT)
Hospitalized patients
FOREST PLOTS -2022-10-14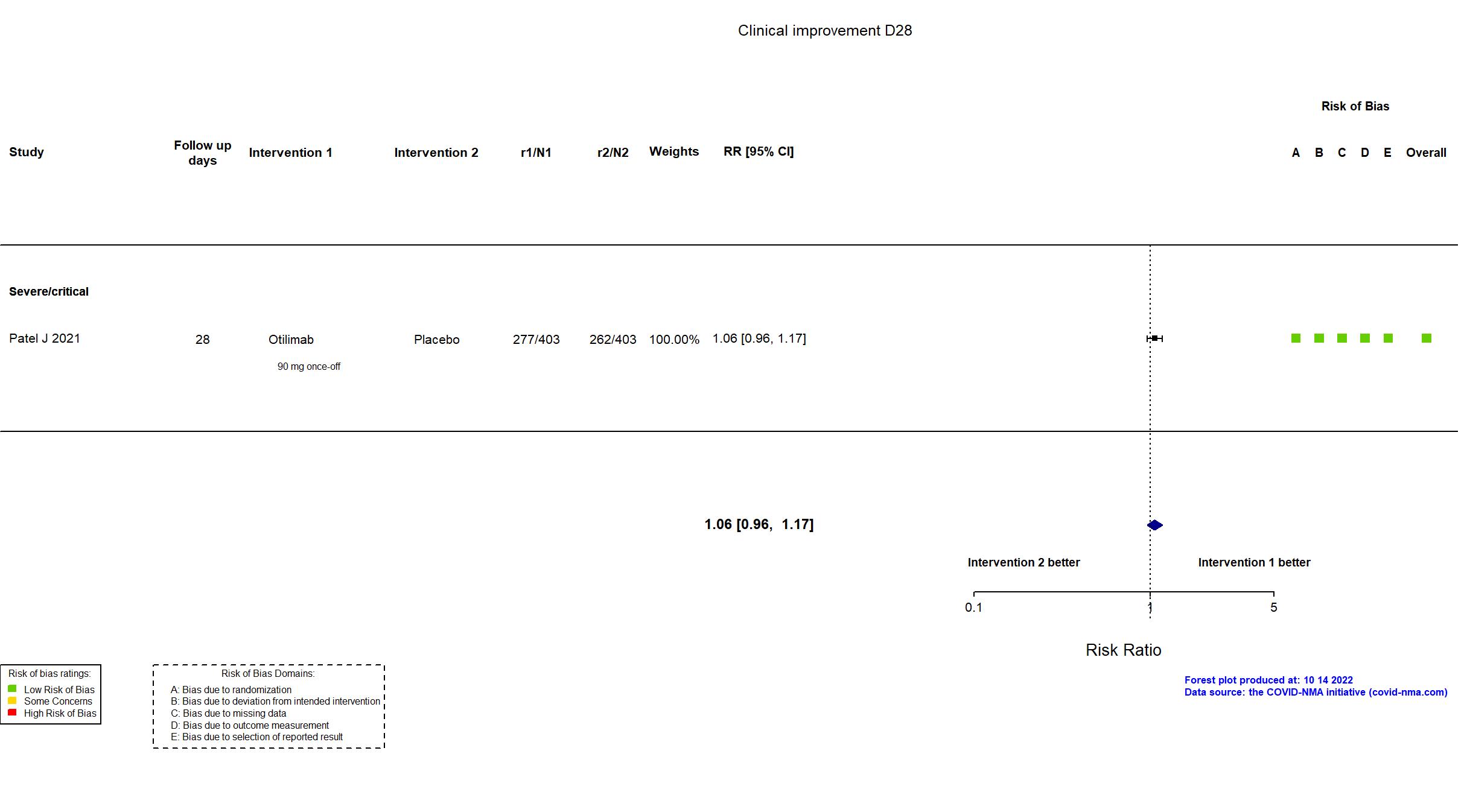

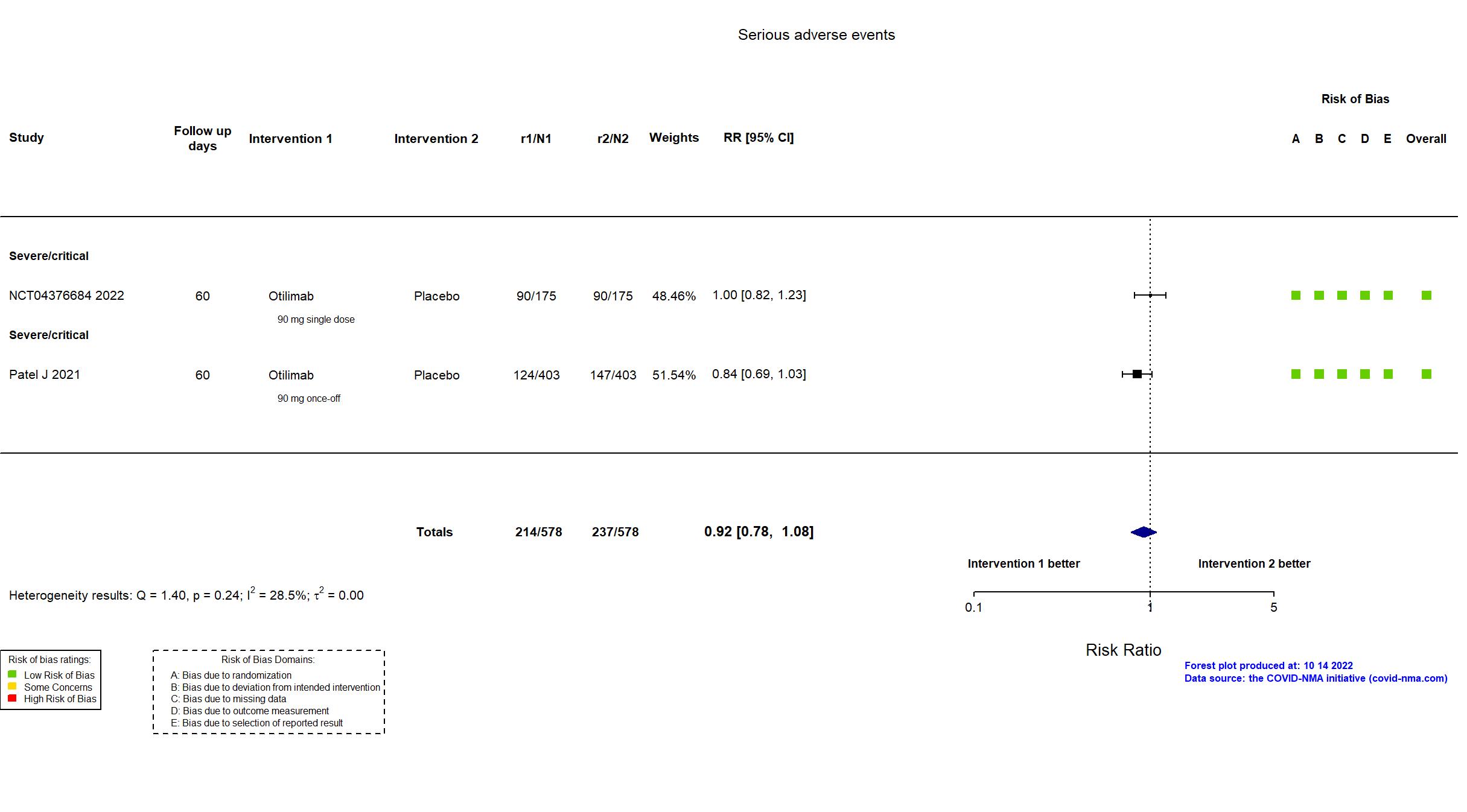
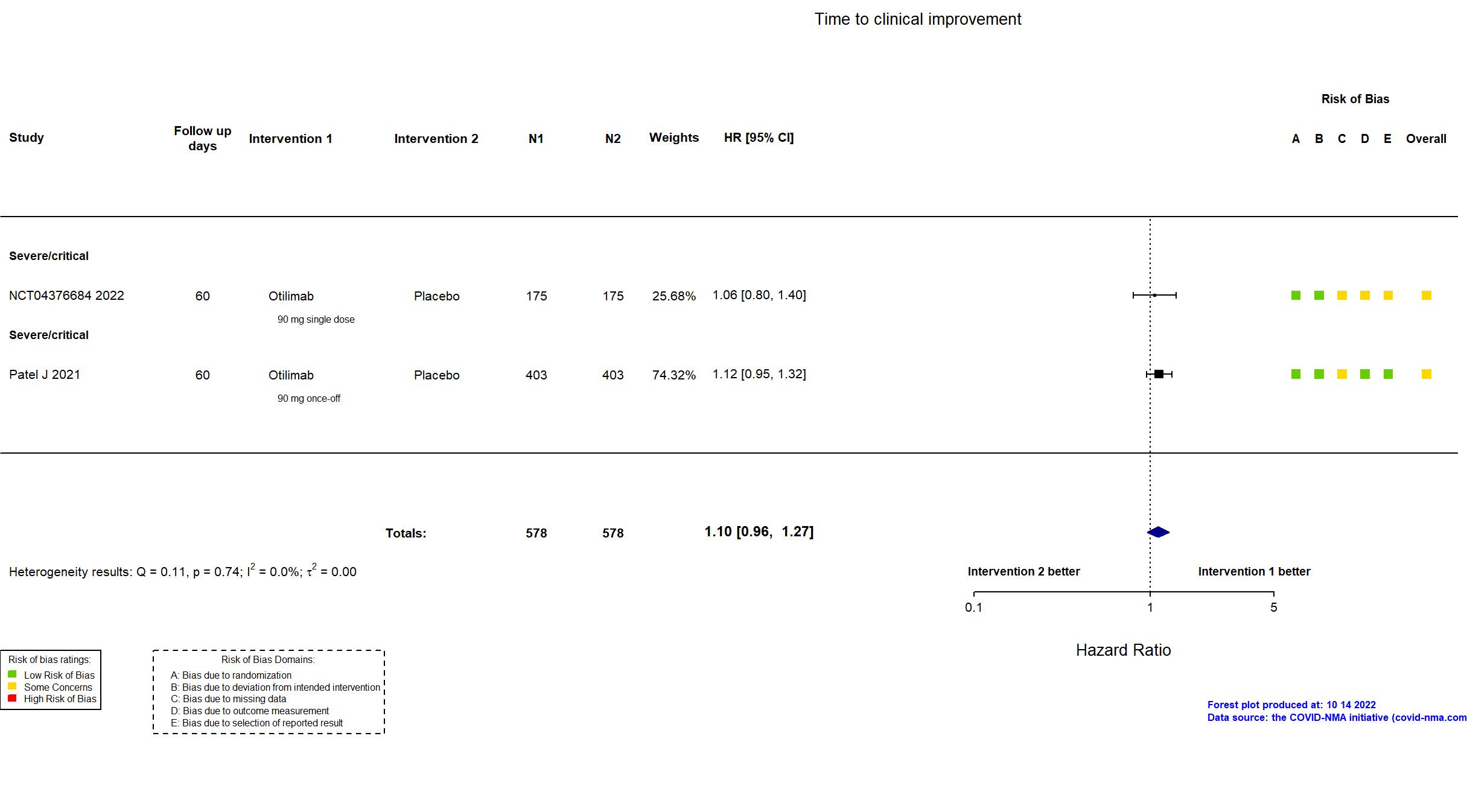
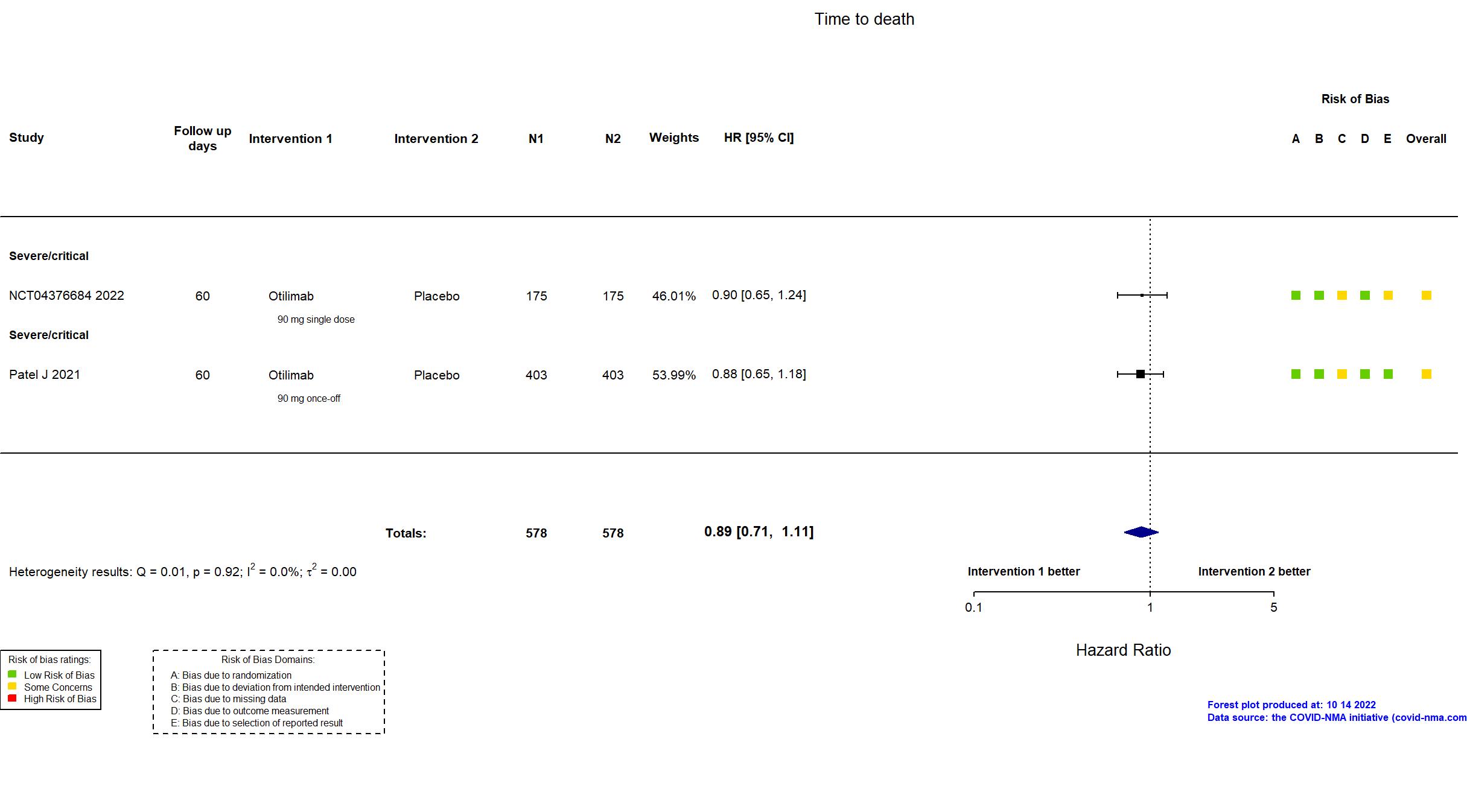








Trial NCT04376684
Publication OSCAR - NCT04376684, Unpublished (2022) (results posted on registry)
Funding: Not reported/unclear
Conflict of interest: *
| Methods | |
| RCT Blinding: double blinding | |
| Location :
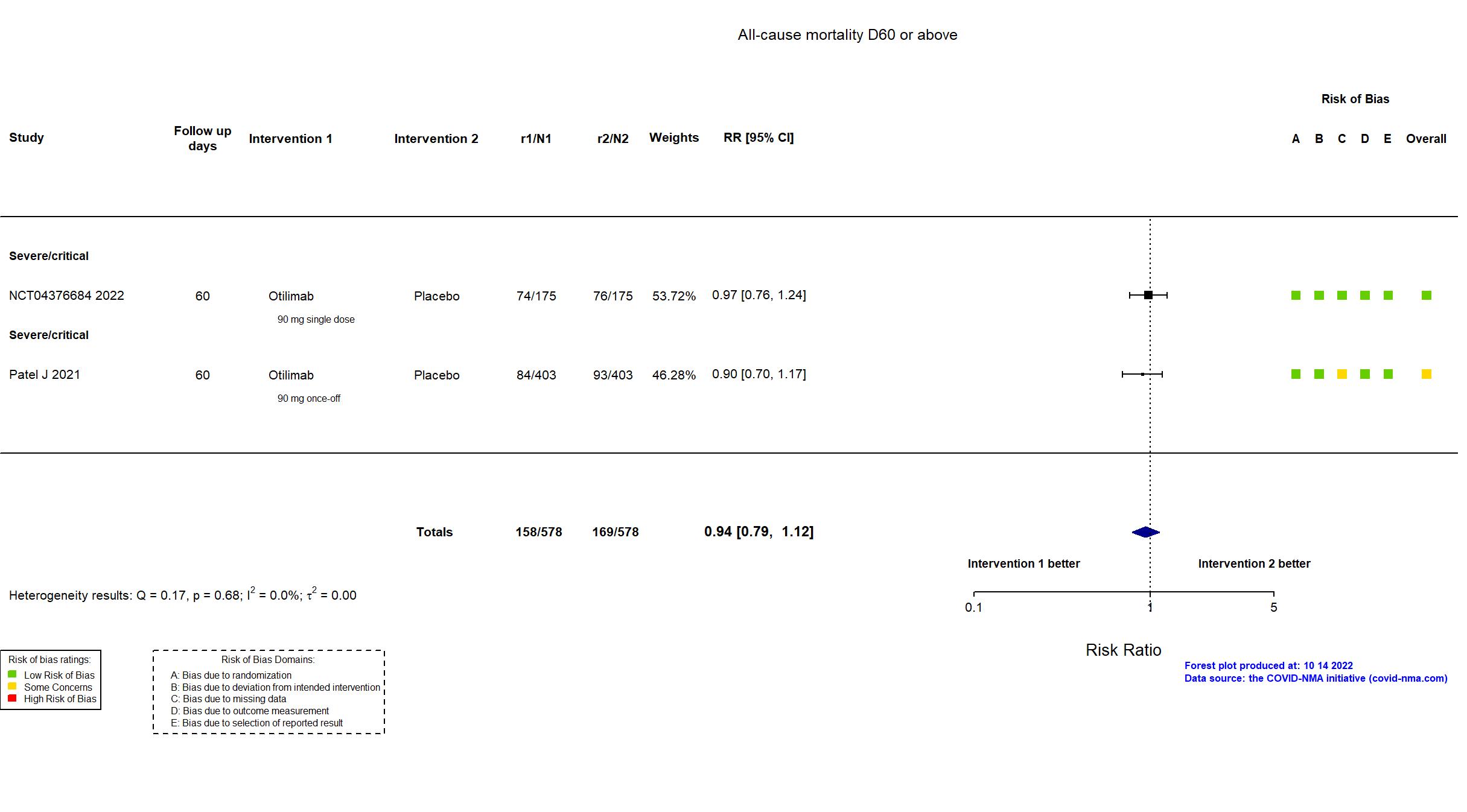
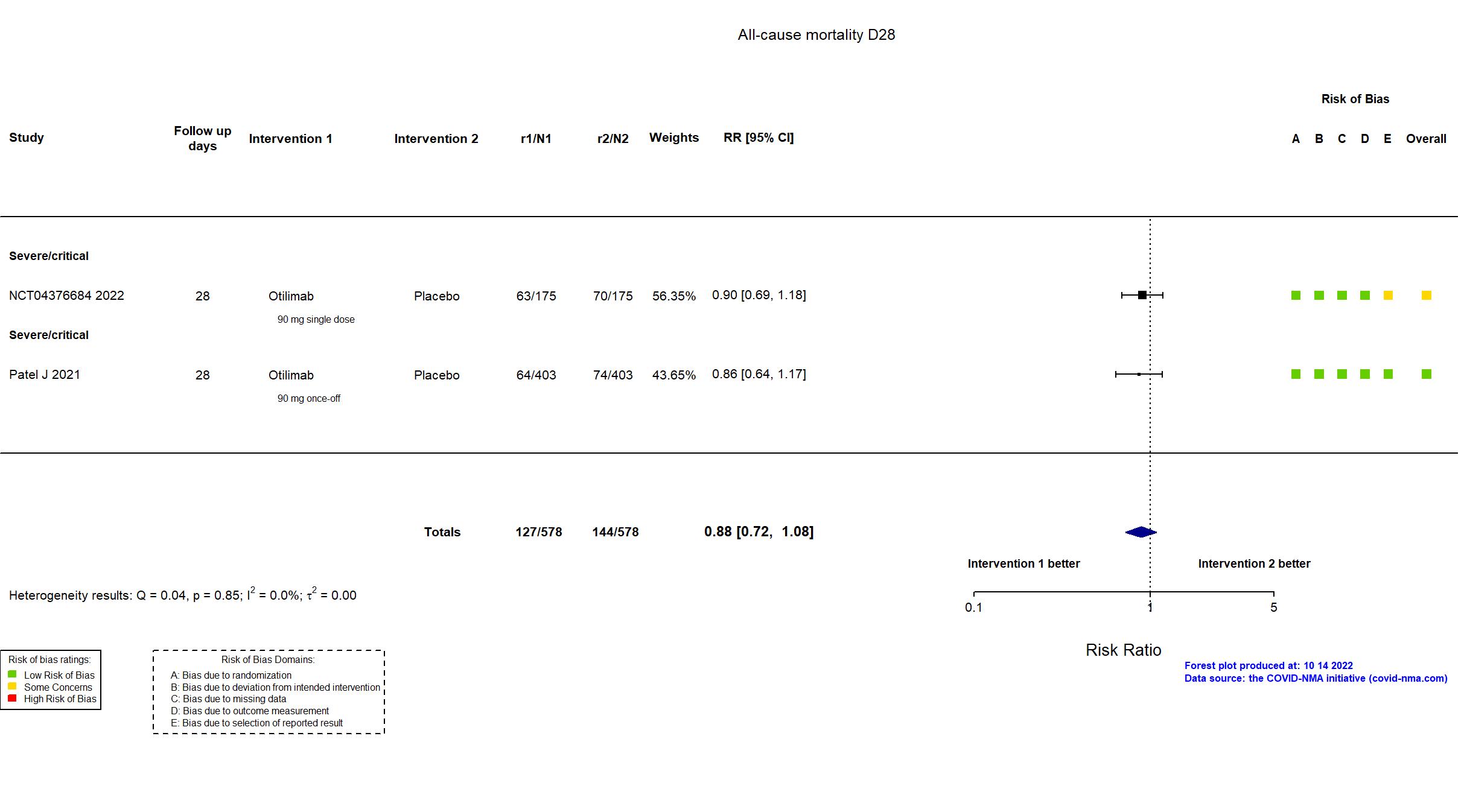
Multicenter / Argentina, Belgium, Brazil, Canada, Chile, Colombia, France, India, Italy, Japan, Mexico, Netherlands, Peru, Poland, Russia, South Africa, Spain, UK, USA Follow-up duration (days): 60 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Otilimab 90 mg IV infusion single dose for 1 hour |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Placebo=175 Otilimab=175 | |
| Characteristics of participants N= 350 Mean age : NR 202 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=* Critical: n=* | |
| Primary outcome | |
| In the register Percentage of Participants Alive and Free of Respiratory Failure at Day 28 [ Time Frame: At Day 28 ] Participants were alive and free of respiratory failure if they were in category 1, 2, 3 or 4 from the GlaxoSmithKline (GSK) modified version ordinal scale adapted from World Health Organization (WHO) scale 2020. The 8-point scale was as follows: 1) Non-hospitalized, no limitation of activity; 2) Non-hospitalized,limitation of activity; 3) Hospitalized, no oxygen therapy; 4) Hospitalized, low-flow oxygen by mask or nasal prongs; 5) Hospitalized, highflow oxygen (>=15L/min), continuous positive airway pressure (CPAP), bilevel positive airway pressure (BIPAP), non-invasive ventilation; 6) Hospitalized, intubation and mechanical ventilation; 7) Hospitalized, mechanical ventilation plus additional organ support; 8) Death. Higher scale indicates higher intensity of respiratory failure. | |
| In the report NR | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | This is an unpublished trial whose results have been reported in ClinicalTrials.gov. The trial registry, protocol and statistical analysis plan were used in data extraction and assessment of risk of bias. The trial was registered prospectively. The report is for part 2 (phase 3) of a 2-part study. This additional cohort was added to confirm the efficacy and safety in part 1 (phase 2). |
Trial NCT04376684
Publication OSCAR - Patel J, medRxiv (2021) (preprint)
Dates: 2020-05-28 to 2020-11-15
Funding: Private (GSK)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / Argentina, Belgium, Brazil, Canada, Chile, France, India, Japan, Mexico, Netherlands, Peru, Poland, Russia, South Africa, Spain, UK, USA Follow-up duration (days): 60 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Otilimab 90 mg single (1 hour) intravenous infusion |
|
| Control
Placebo | |
| Participants | |
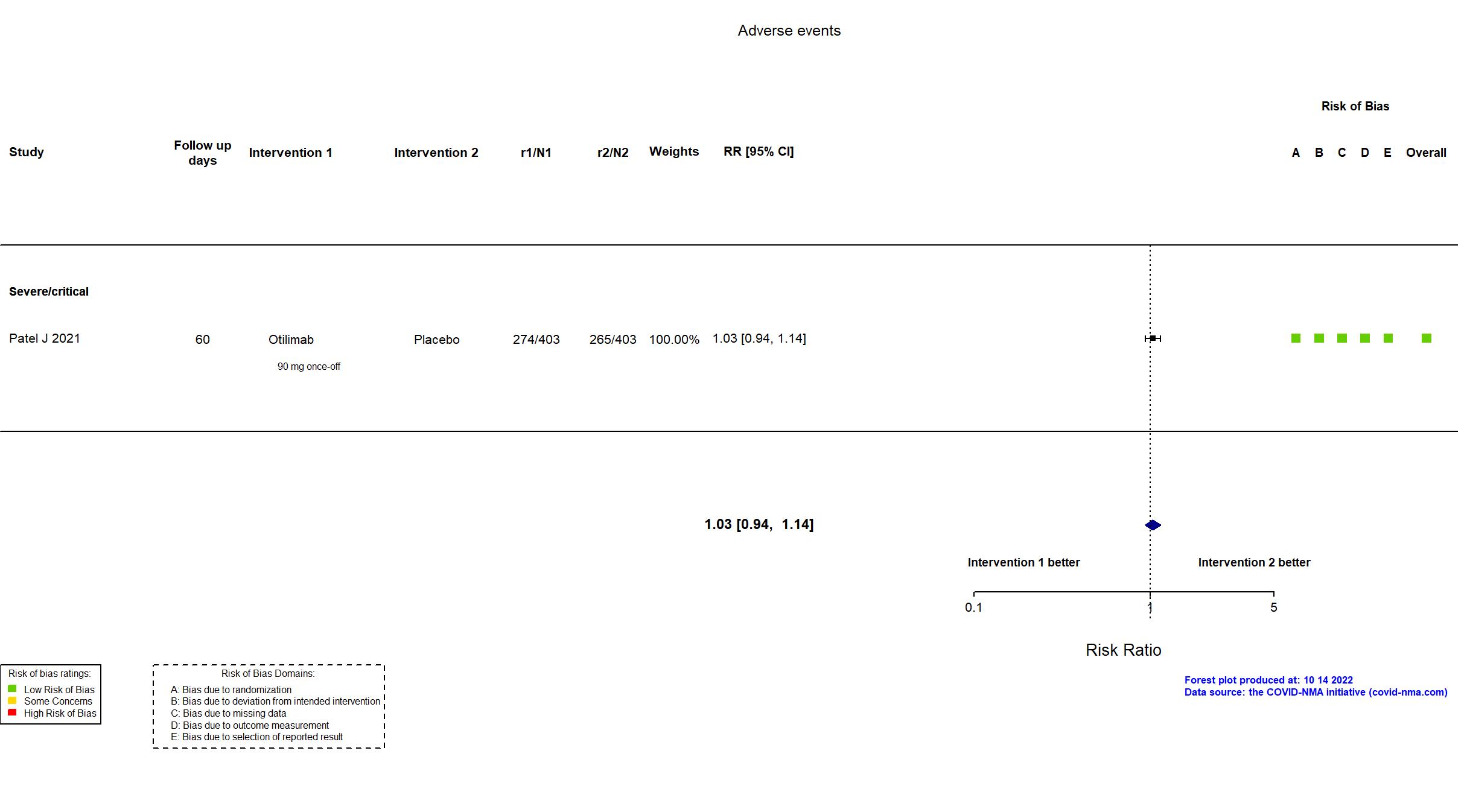
| Randomized participants : Otilimab=403 Placebo=403 | |
| Characteristics of participants N= 806 Mean age : NR 577 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=622 Critical: n=178 | |
| Primary outcome | |
| In the register Proportion of participants alive and free of respiratory failure at Day 28 (measured by an 8-point ordinal scale). | |
| In the report The proportion of patients alive and free of respiratory failure (clinical status Categories 1, 2, 3, or 4) at Day 28 | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the pre-print article, the trial registry, protocol and statistical analysis plan were used in data extraction and assessment of risk of bias. There were no substantive differences between the article and the trial registry, protocol and statistical analysis plan in population, procedures, intervention and outcomes. The primary outcome indicated in the registry reflects the primary outcome reported in the paper. The report is for part 1 of a 2-part study. The target sample size in the trial registry appears to be for both parts 1 and 2. Part 1 achieved the sample size specified in the protocol and methods section.
This study was updated on the 28th of September 2022 with data extracted from the registry. |