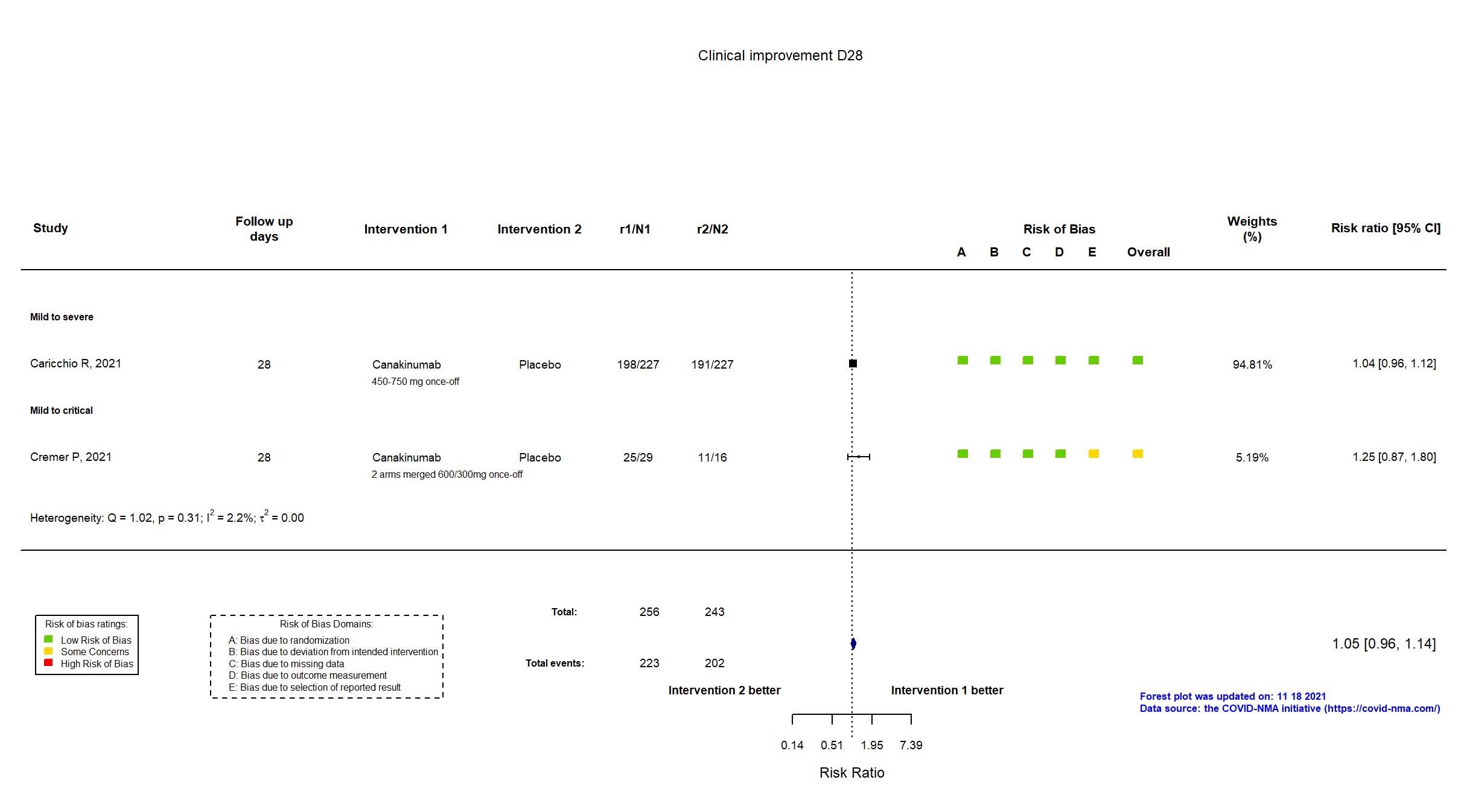
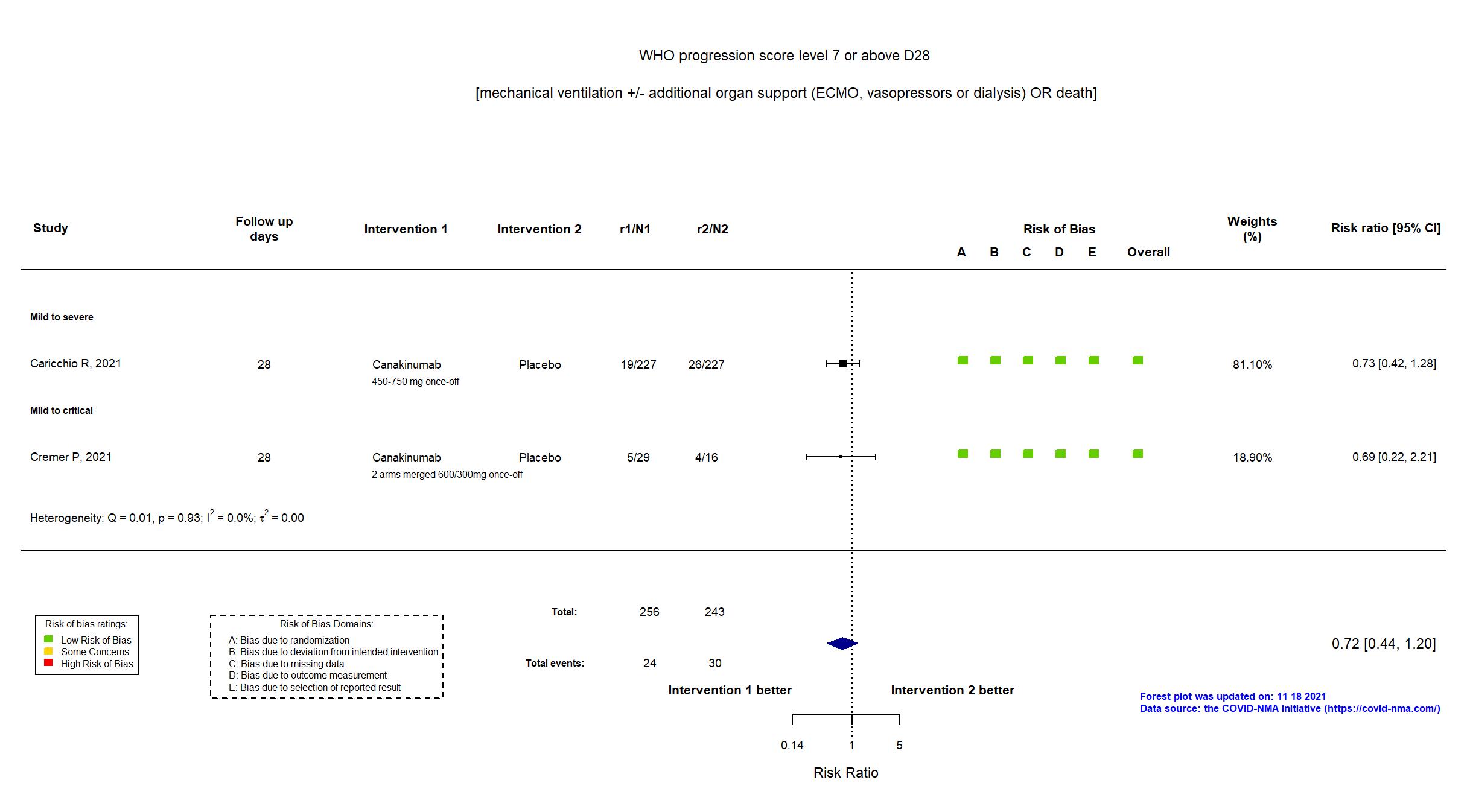
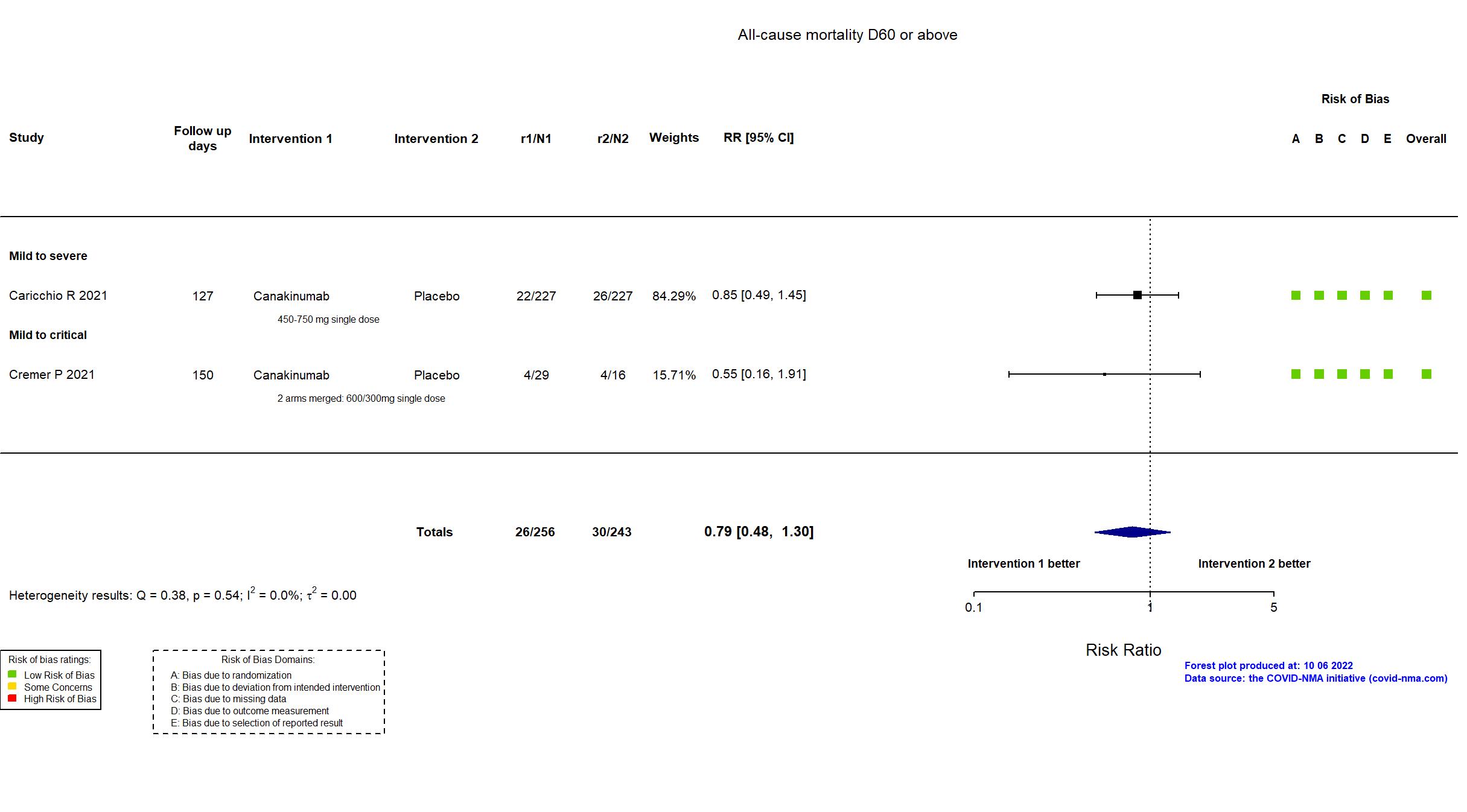
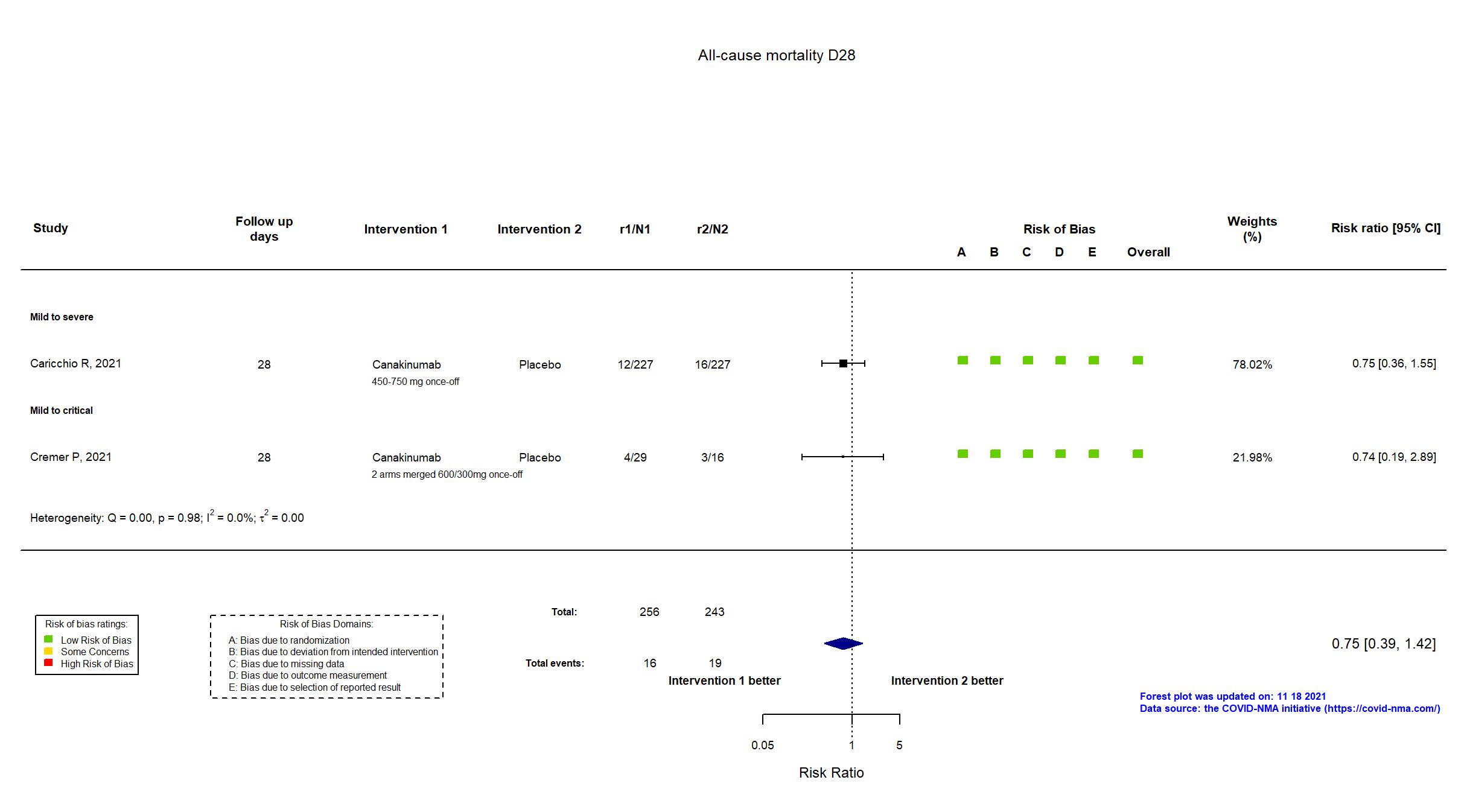
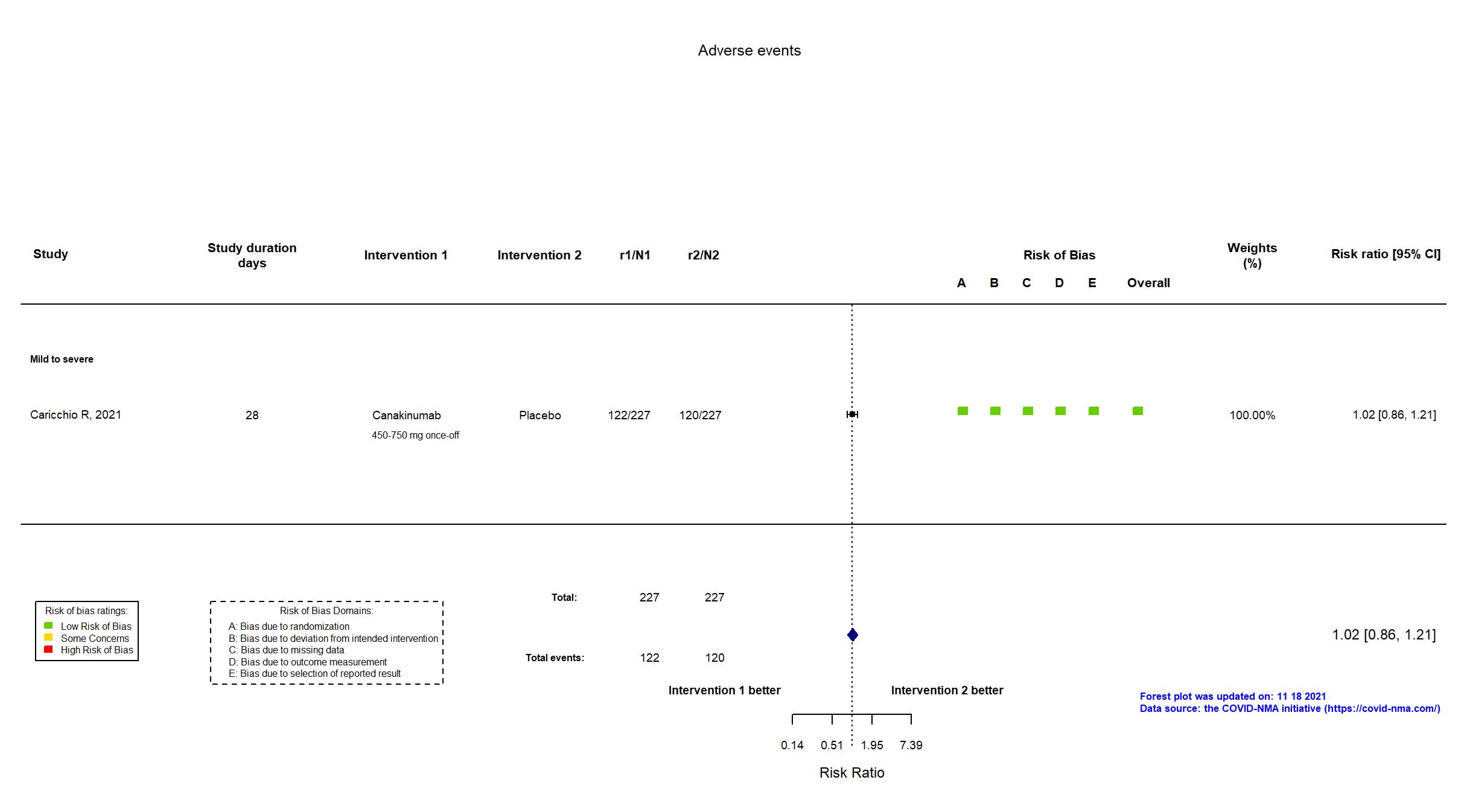
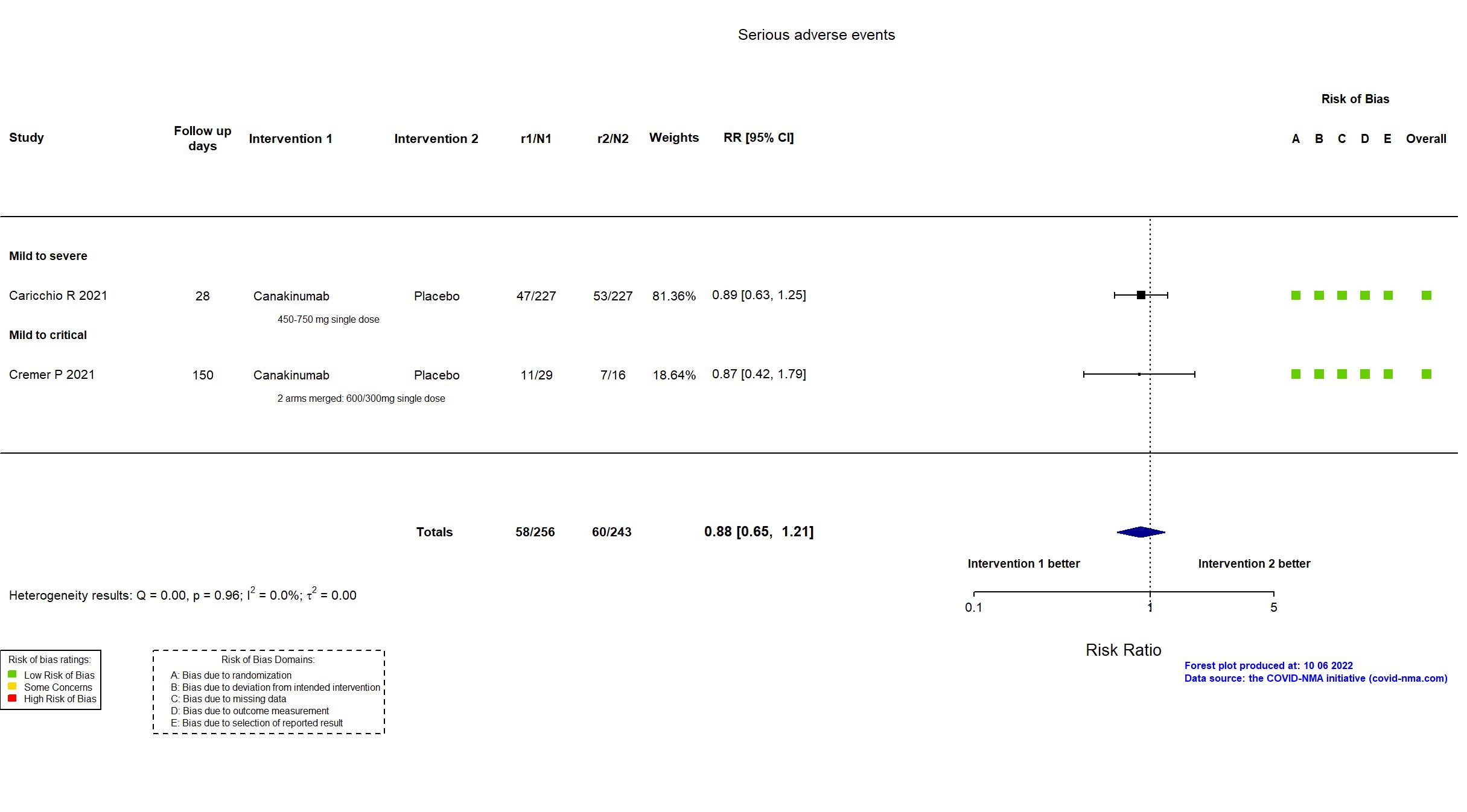
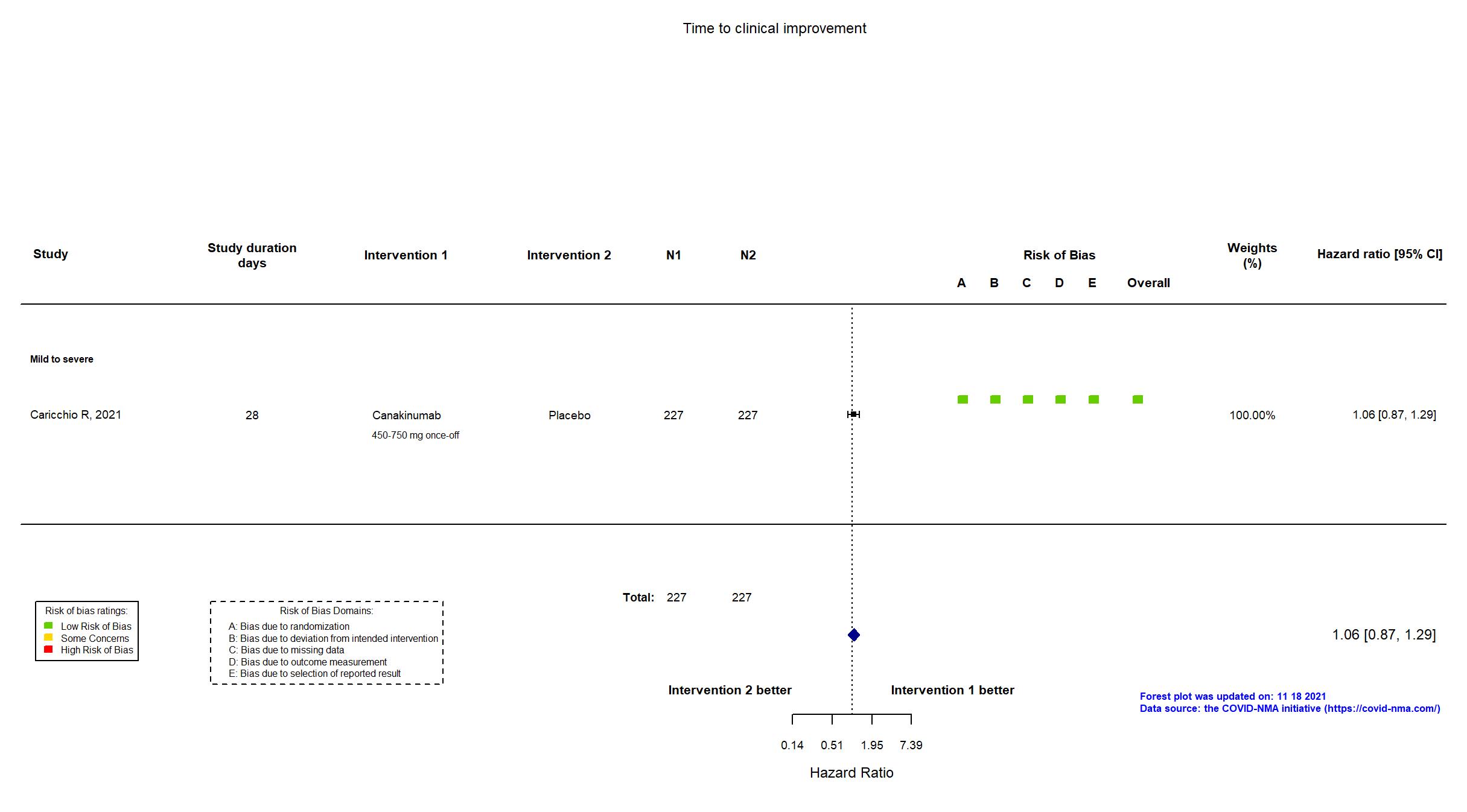
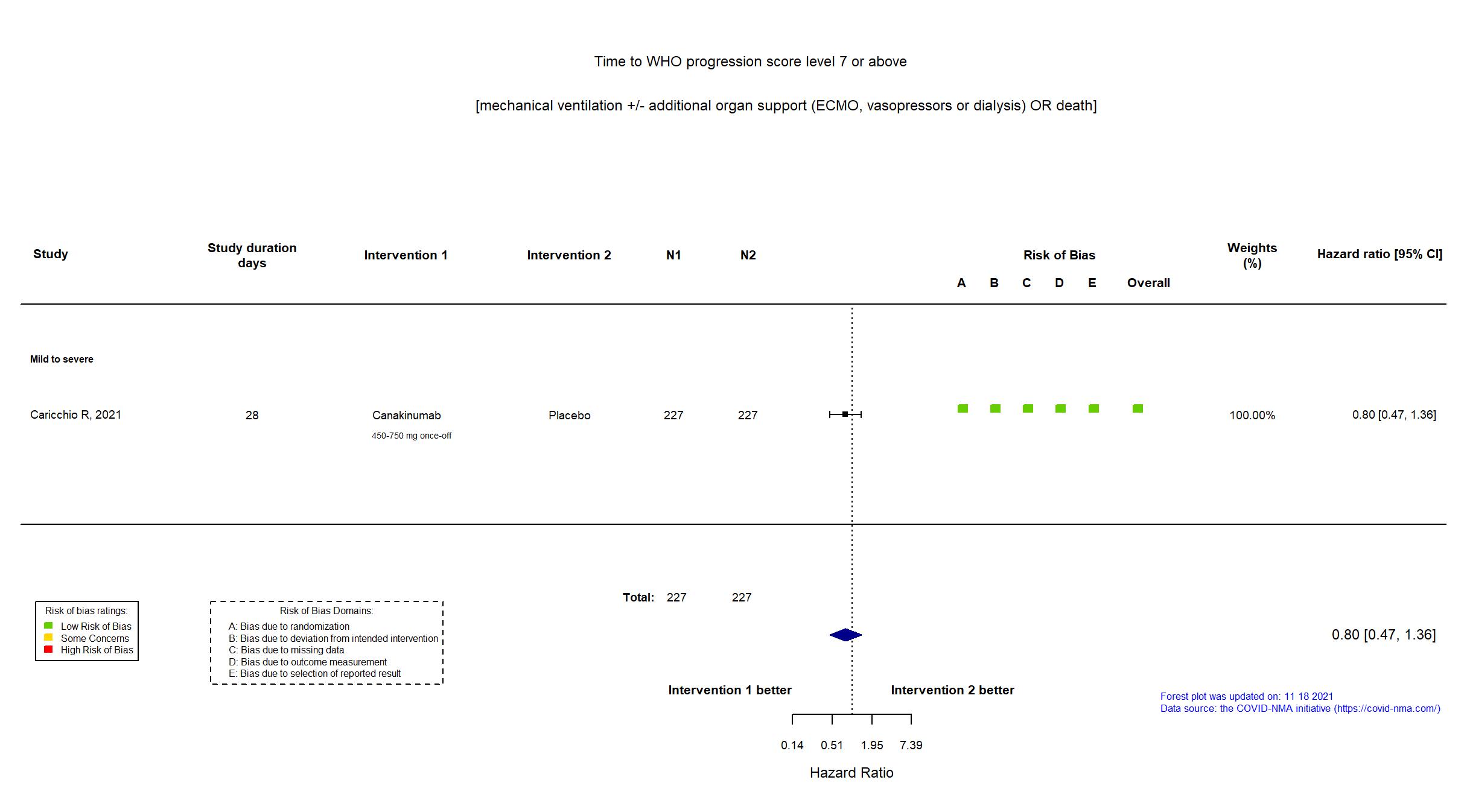
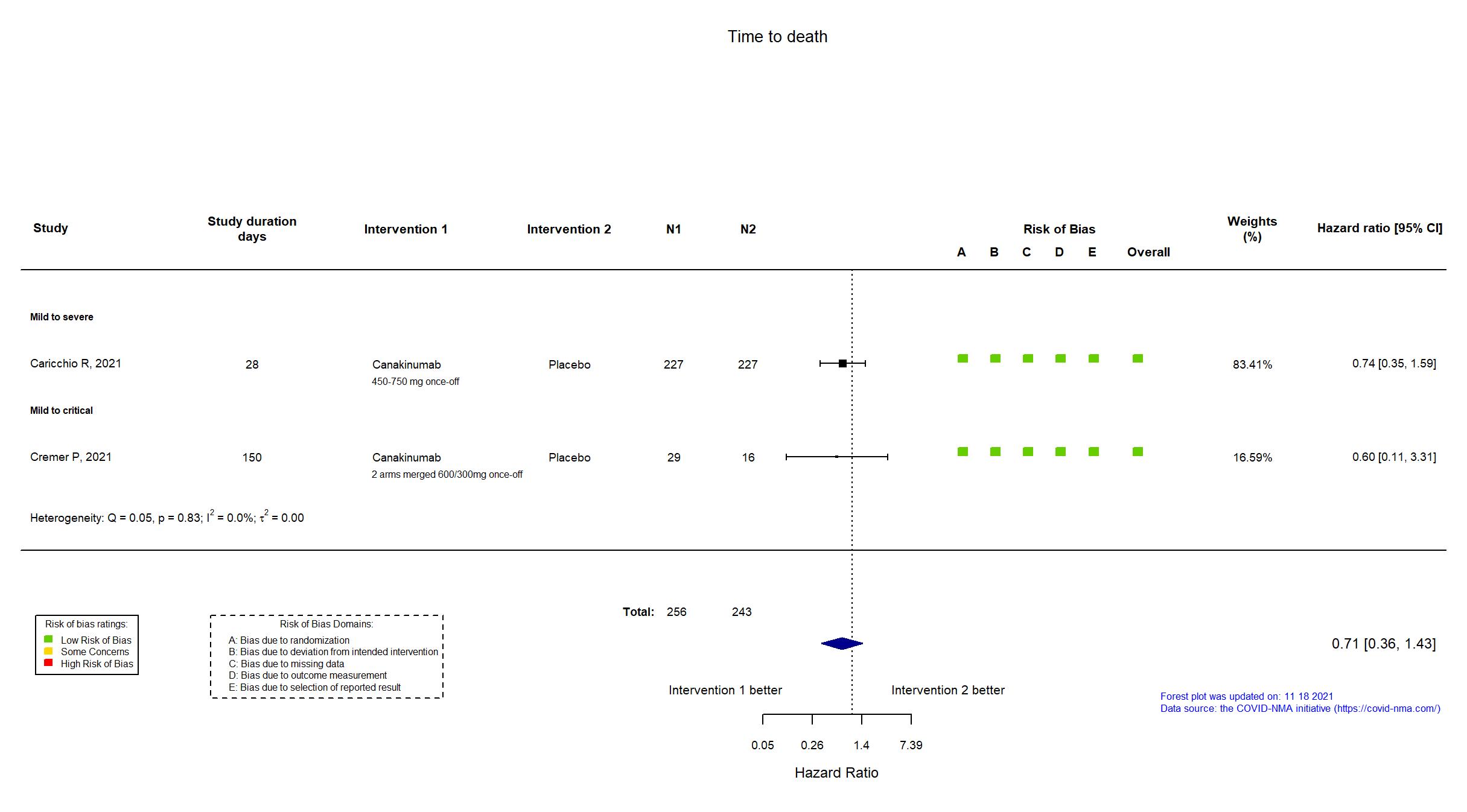
Canakinumab vs Placebo (RCT)
Hospitalized patients
On Jan 26th, 2022, we published results of the Are medicines that block interleukin‐1 (a protein involved in immune responses) effective treatments for COVID‐19 and do they cause unwanted effects? which included all preprints and published trials published online up to November 5, 2021.
Studies included but not extracted/included in the analysis: Hepprich M, EClinicalMedicine, 2022
FOREST PLOTS -2022-10-07