Regdanvimab (CT-P59) vs Placebo (RCT)
Mild outpatients
FOREST PLOTS -2022-11-22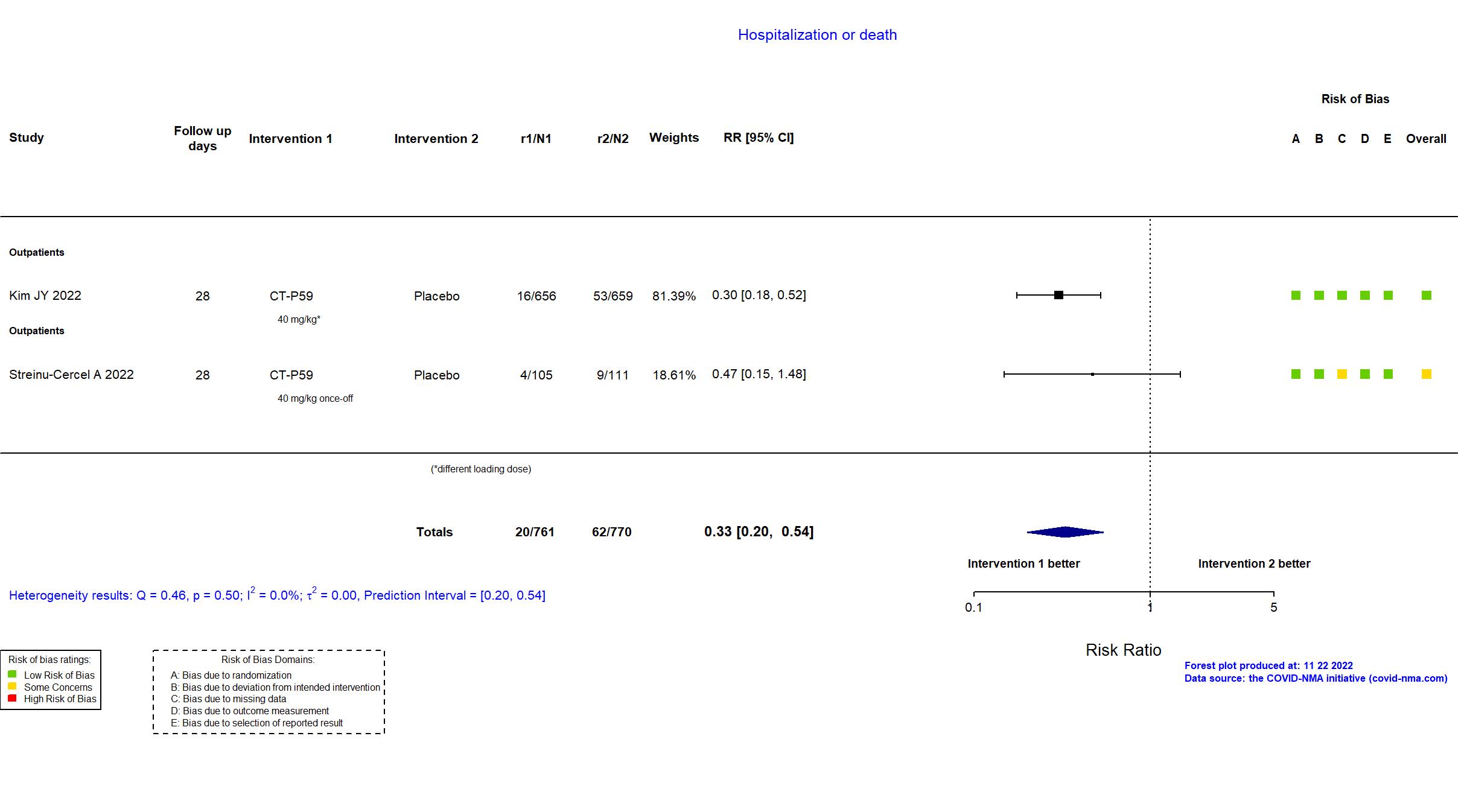
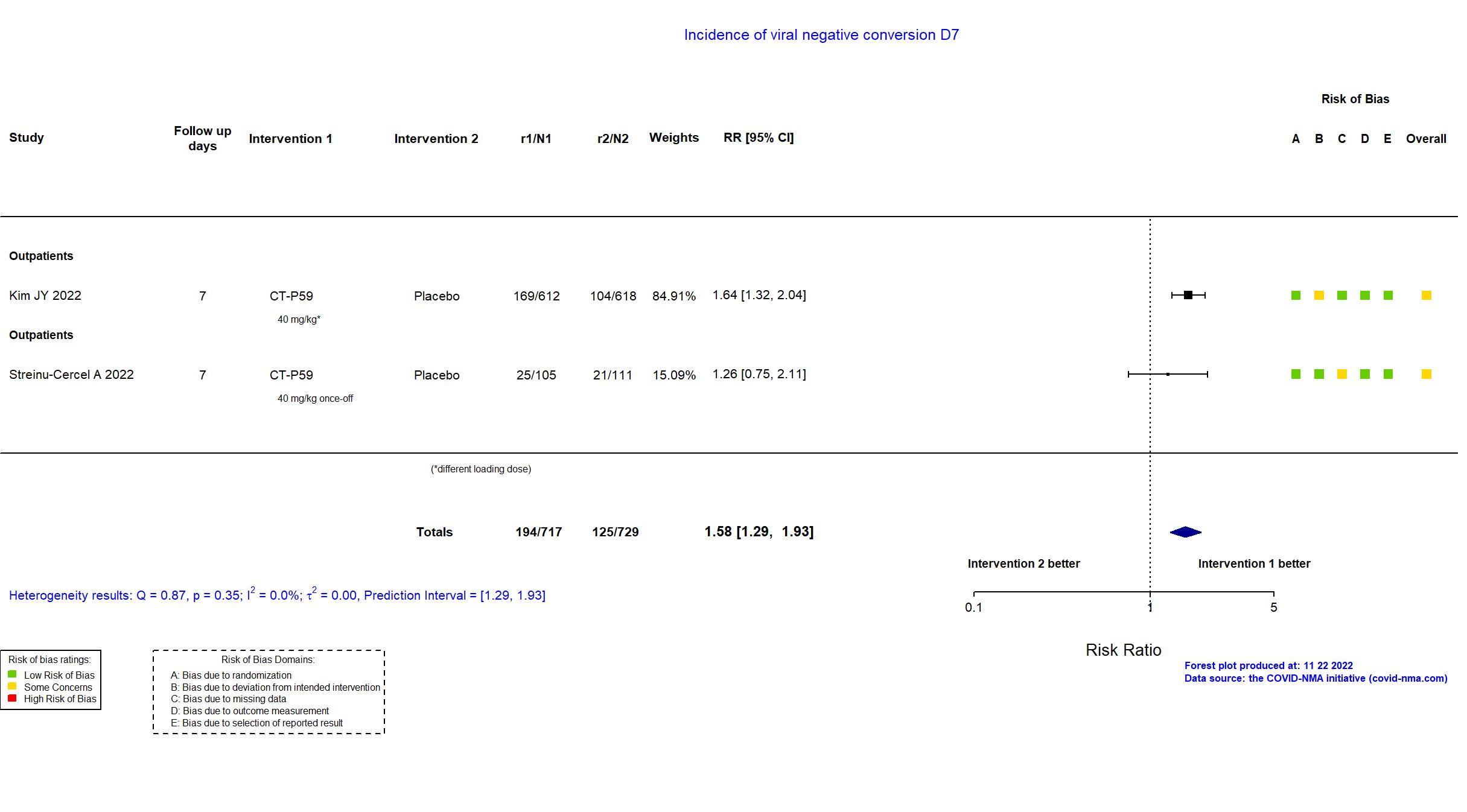
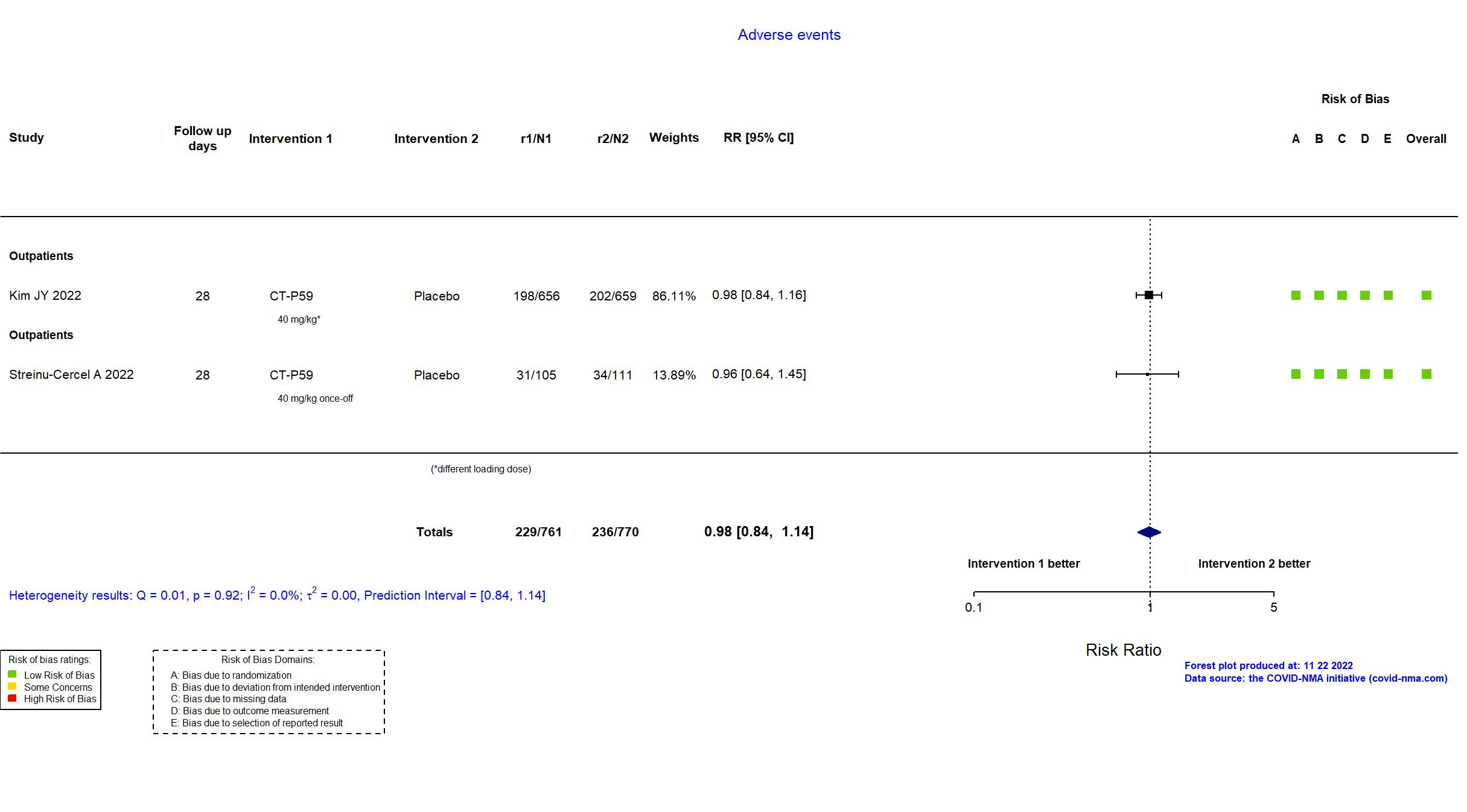
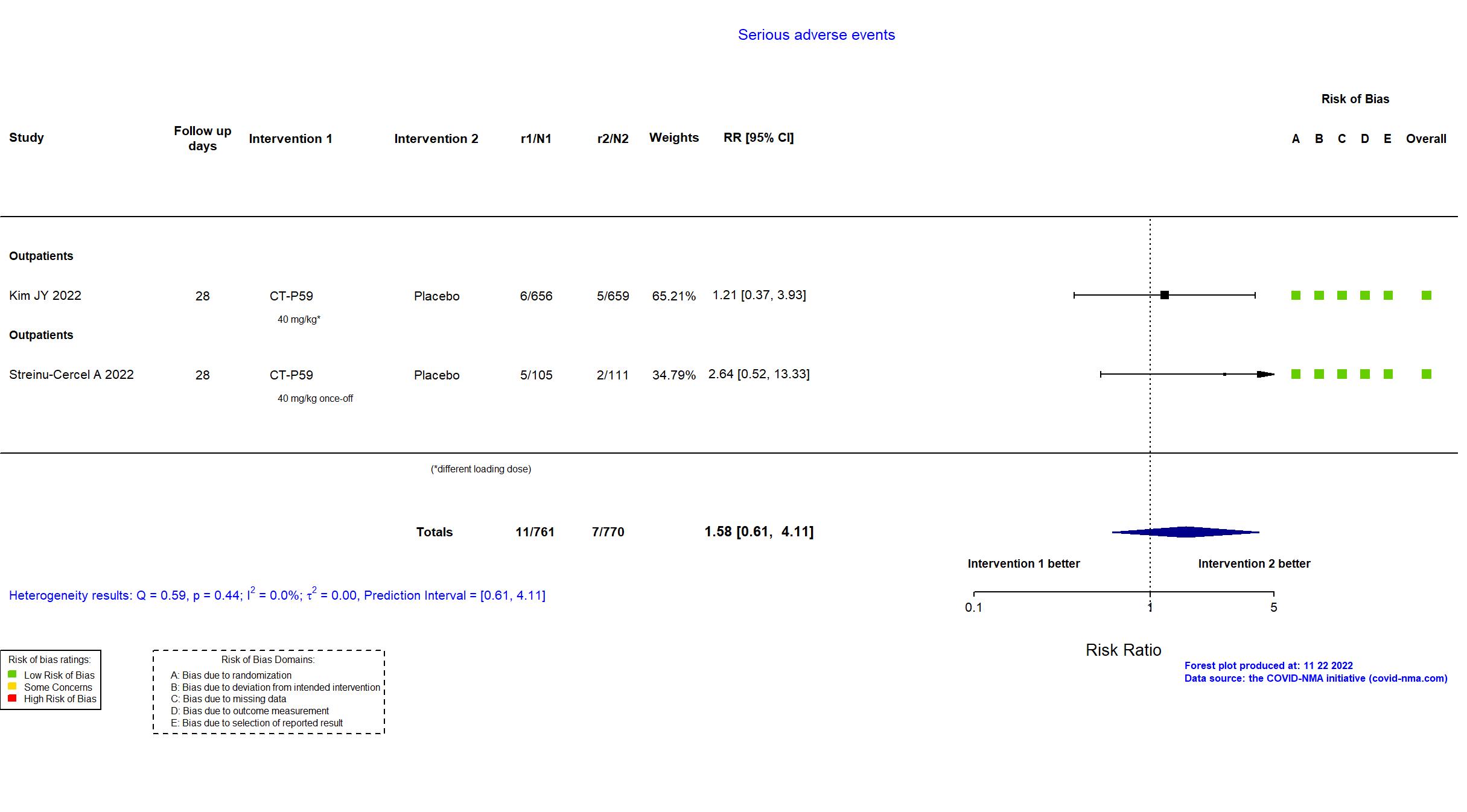







Trial NCT04602000; EudraCT 2020-003369-20
Publication Kim JY, Open Forum Infect Dis (2022) (published paper)
Dates: 2021-01-18 to 2021-04-24
Funding: Mixed (Celltrion, Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, South Korea)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: quadruple blinding | |
| Location :
Multicenter / Hungary, Ireland, Italy, Macedonia, Mexico, Moldova, Peru, Poland, Republic of Korea, Romania, Serbia, Spain, Ukraine, USA Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
CT-P59 40 mg/kg single dose by intravenous infusion over 60 +/- 15 minutes |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Placebo=659 CT-P59=656 | |
| Characteristics of participants N= 1315 Mean age : NR 674 males Severity : Mild: n= 1315/ Asymptomatic: n=0 | |
| Primary outcome | |
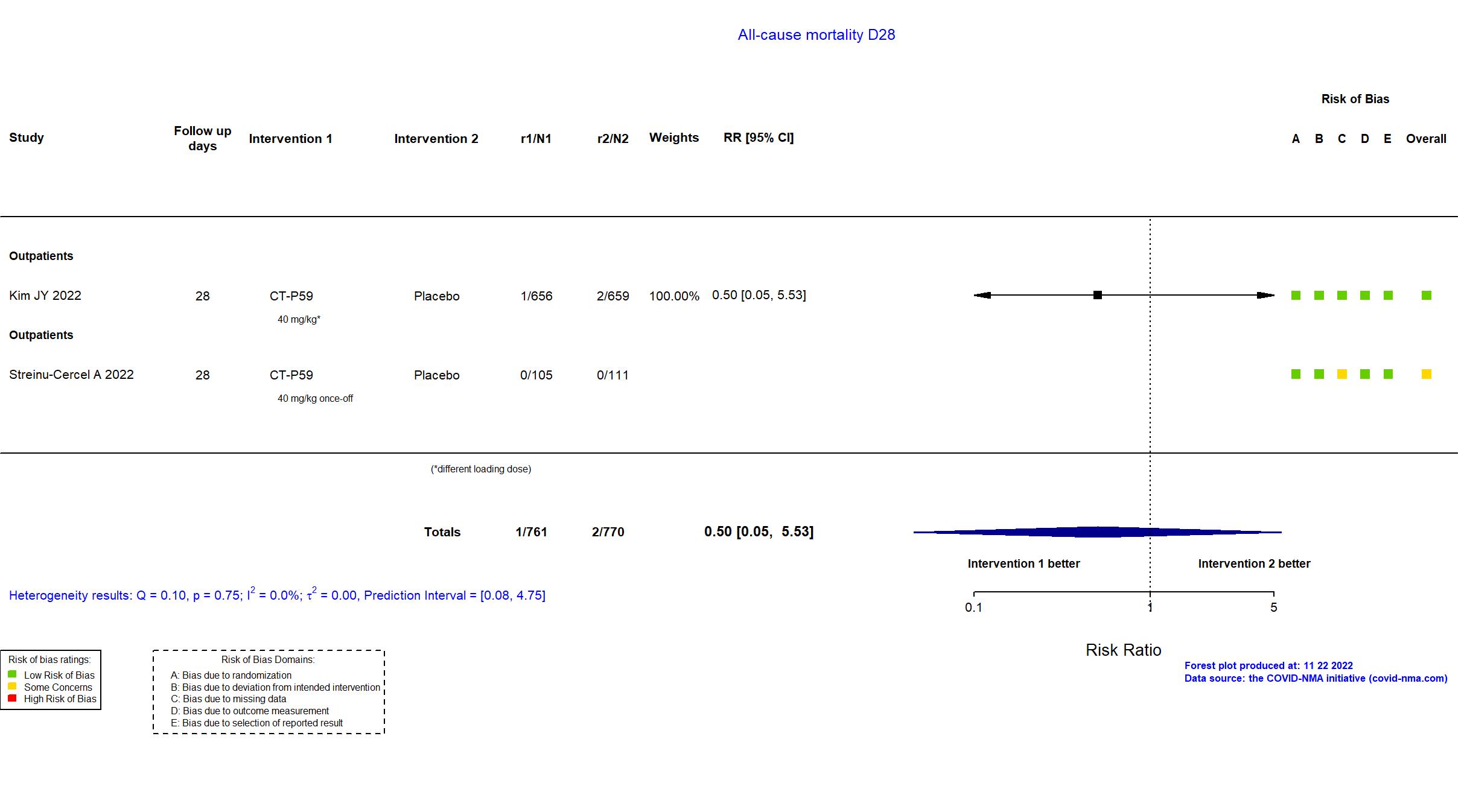
| In the register 1) Proportion of Patients With Clinical Symptom Requiring Hospitalization, Oxygen Therapy, or Experiencing Mortality Due to SARS-CoV-2 Infection (Part 1) [ Time Frame: Up to Day 28]; 2) Proportion of Patients With Negative Conversion in Nasopharyngeal Swab Specimen Based on RT-qPCR at Each Visit (Part 1) [ Time Frame: Up to Day 14 ]; 3) Time to Negative Conversion in Nasopharyngeal Swab Specimen (Part 1) [ Time Frame: Up to Day 14 ]; 4) Time to Clinical Recovery (Part 1) [ Time Frame: Up to Day 14 ]; 5) Proportion of Patients With Clinical Symptom Requiring Hospitalization, Oxygen Therapy, or Experiencing Mortality Due to SARS-CoV-2 Infection up to Day 28 in High-risk Patients (Part 2) [ Time Frame: Up to Day 28 ] | |
| In the report Proportion of patients with disease progression up to day 28 in high-risk patients. Disease progression was defined as meeting at least 1 of the following COVID-19 events: hospitalization, oxygen therapy, or mortality due to SARS-CoV-2 infection. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: N |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the protocol, statistical analysis plan, supplemental material and study registry were used in data extraction and risk of bias assessment. There is no change from the trial registration in the intervention and control treatments. The registry had additional primary outcomes that were reported in the paper as secondary outcomes. The study (n=1315) achieved the target sample size specified in the trial registry (n=1020). Of note, the outcome Hospitalisation or death also included participants who received oxygen therapy without specifying if they were hospitalized or not. |
Trial NCT04602000, EudraCT: 2020-003369-20
Publication Streinu-Cercel A, Open Forum Infect Dis (2022) (published paper)
Dates: 2020-10-07 to 2020-11-20
Funding: Mixed (Celltrion, Inc; Korea Health Industry Development Institute (KHIDI); Ministry of Health & Welfare, Republic of Korea; Medical writing support was funded by Celltrion, Inc.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: triple blinding | |
| Location :
Multicenter / South Korea, Romania, Spain, USA Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
CT-P59 80mg/kg 80 mg/kg once-off via intravenous infusion over 90 ± 15 minutes CT-P59 40 mg/kg once-off via intravenous infusion over 90 ± 15 minutes |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : CT-P59 80mg/kg=111 CT-P59 =105 Placebo=111 | |
| Characteristics of participants N= 327 Mean age : NR 166 males Severity : Mild: n= 327/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register 1) Proportion of patients with negative conversion in nasopharyngeal swab specimen based on RT-qPCR or cell culture at each visit for Part 1 (Phase II) [ Time Frame: Up to Day 14 ]; 2) Time to negative conversion in nasopharyngeal swab specimen based on RT-qPCR or cell culture at each visit for Part 1 (Phase II) [ Time Frame: Up to Day 14 ] 3) Time to clinical recovery for Part 1 (Phase II) [ Time Frame: Up to Day 14 ] 4) Proportion of patients with clinical symptom requiring hospitalization, oxygen therapy, or experiencing mortality due to SARS-CoV-2 infection for Part 1 (Phase II) [ Time Frame: Up to Day 28 ] | |
| In the report 1)Time to conversion to negative nasopharyngeal swab specimen based on RT-qPCR (negative titer threshold of 2.33 log10 copies/mL) up to day 28; 2)Time to clinical recovery up to day 14. | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: N |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | “In addition to the published article, the supplementary appendix and two study registries were used in data extraction and risk of bias assessment. The protocol and statistical analysis plan were not available. The primary outcomes in the article (time to negative conversion to day 28 and time to clinical recovery to day 14) differed slightly from those in the registries (proportion with negative conversion and time to negative conversion to day 14, time to clinical recovery to day 14 and proportion requiring hospitalization, oxygen or experiencing mortality to day 28). The study (n=327) achieved the target sample size (n=300) calculated in the supplementary appendix." |