Hydroxychloroquine + Azithromycin vs Standard care/Placebo (RCT)
Mild outpatients
FOREST PLOTS -2021-09-24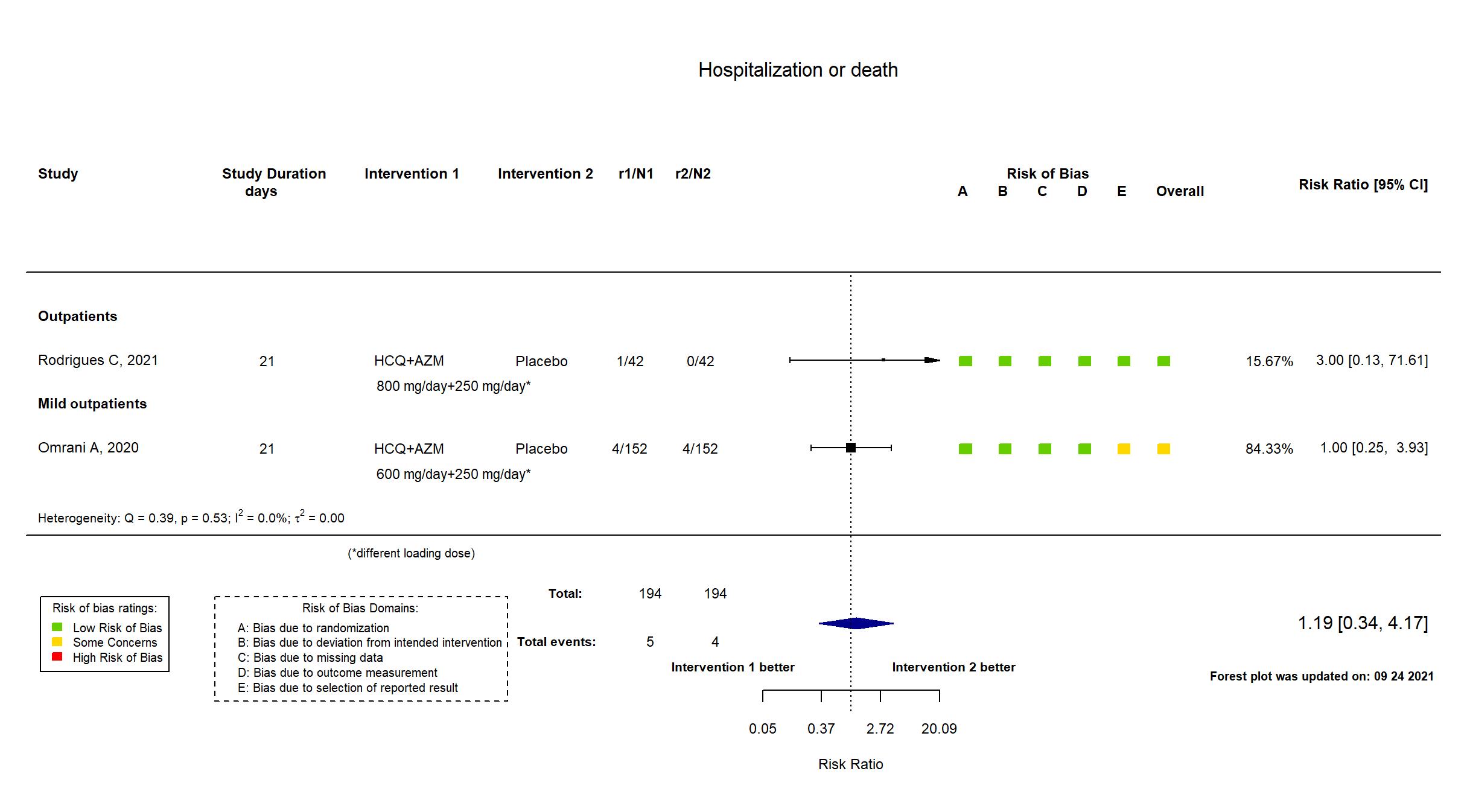
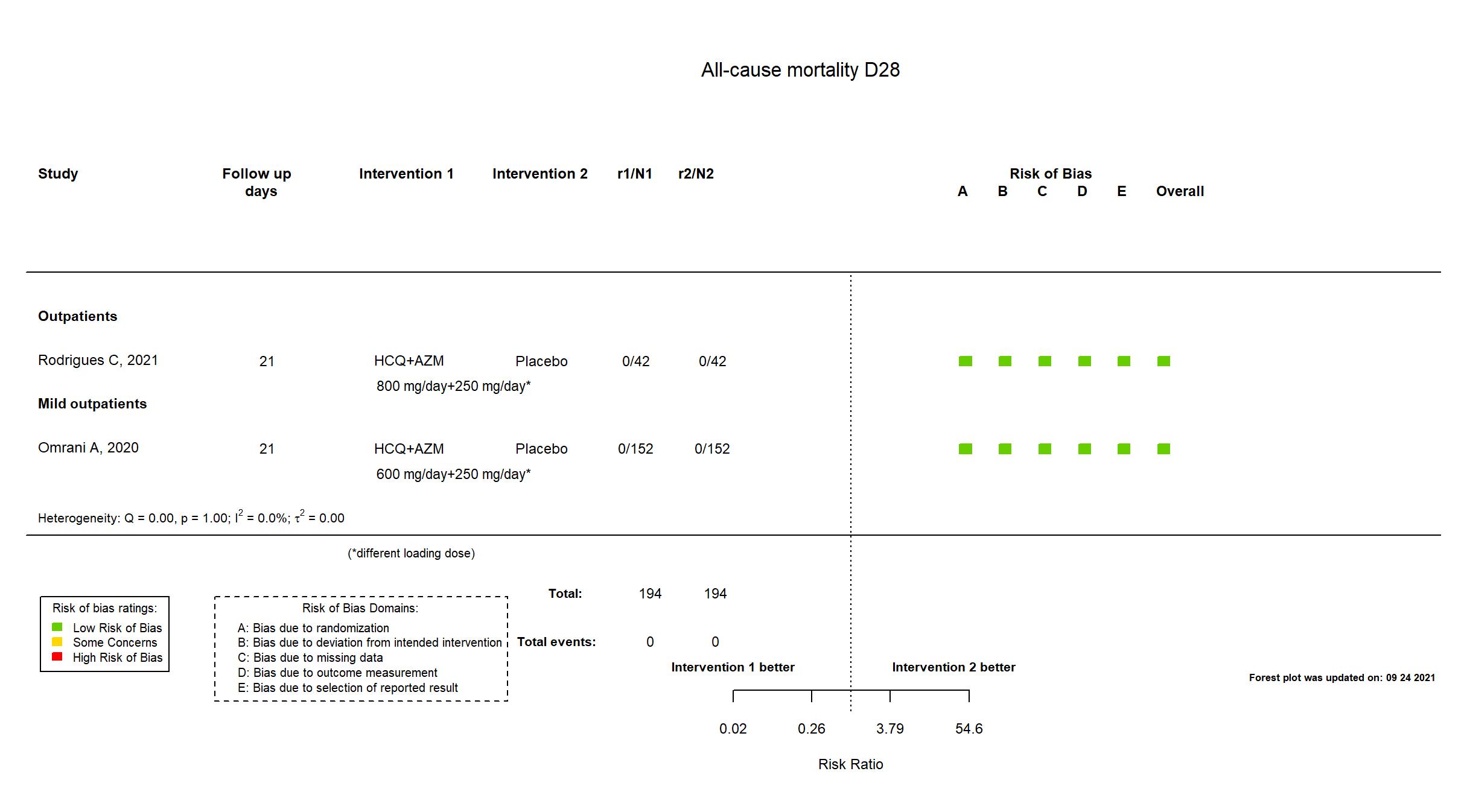
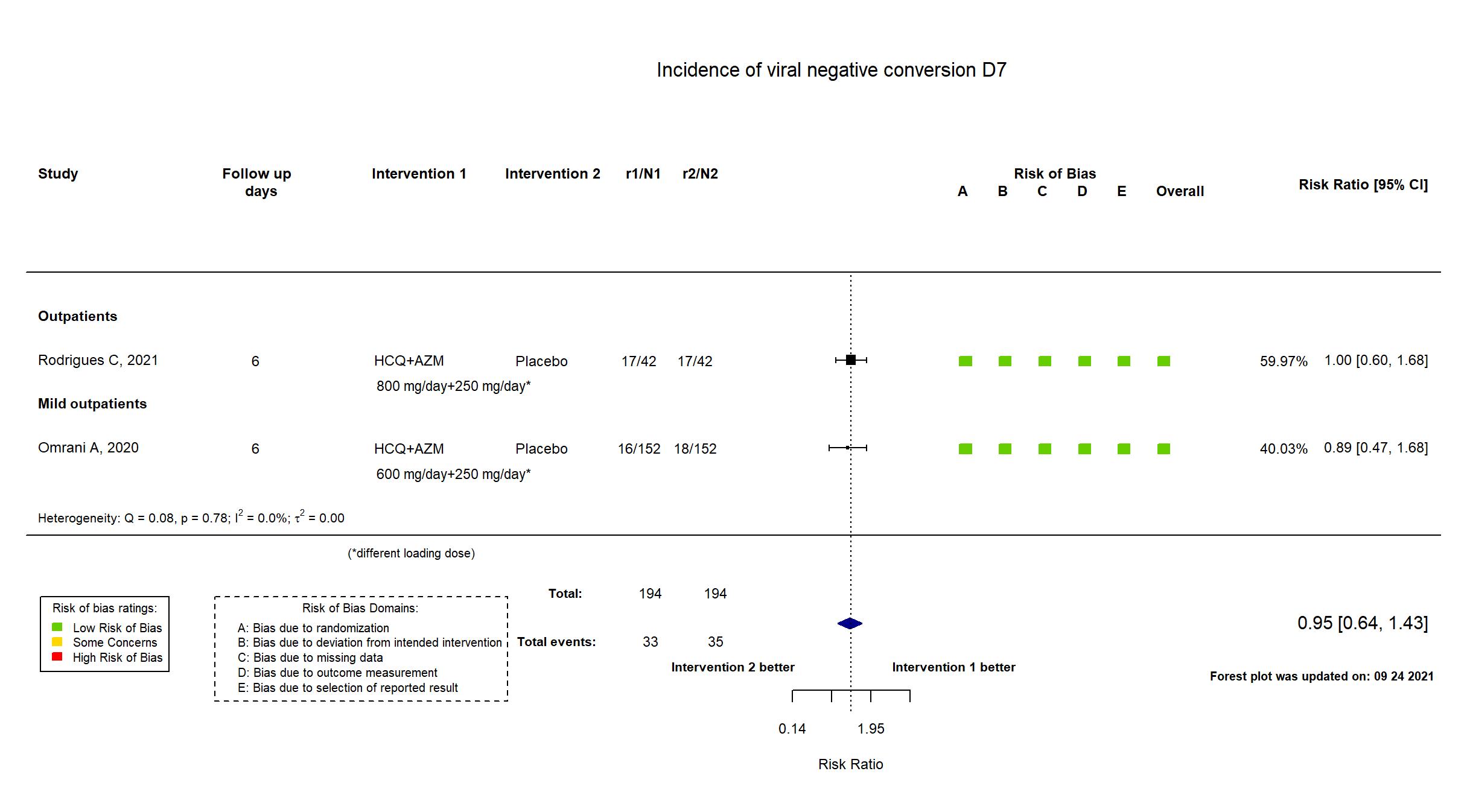
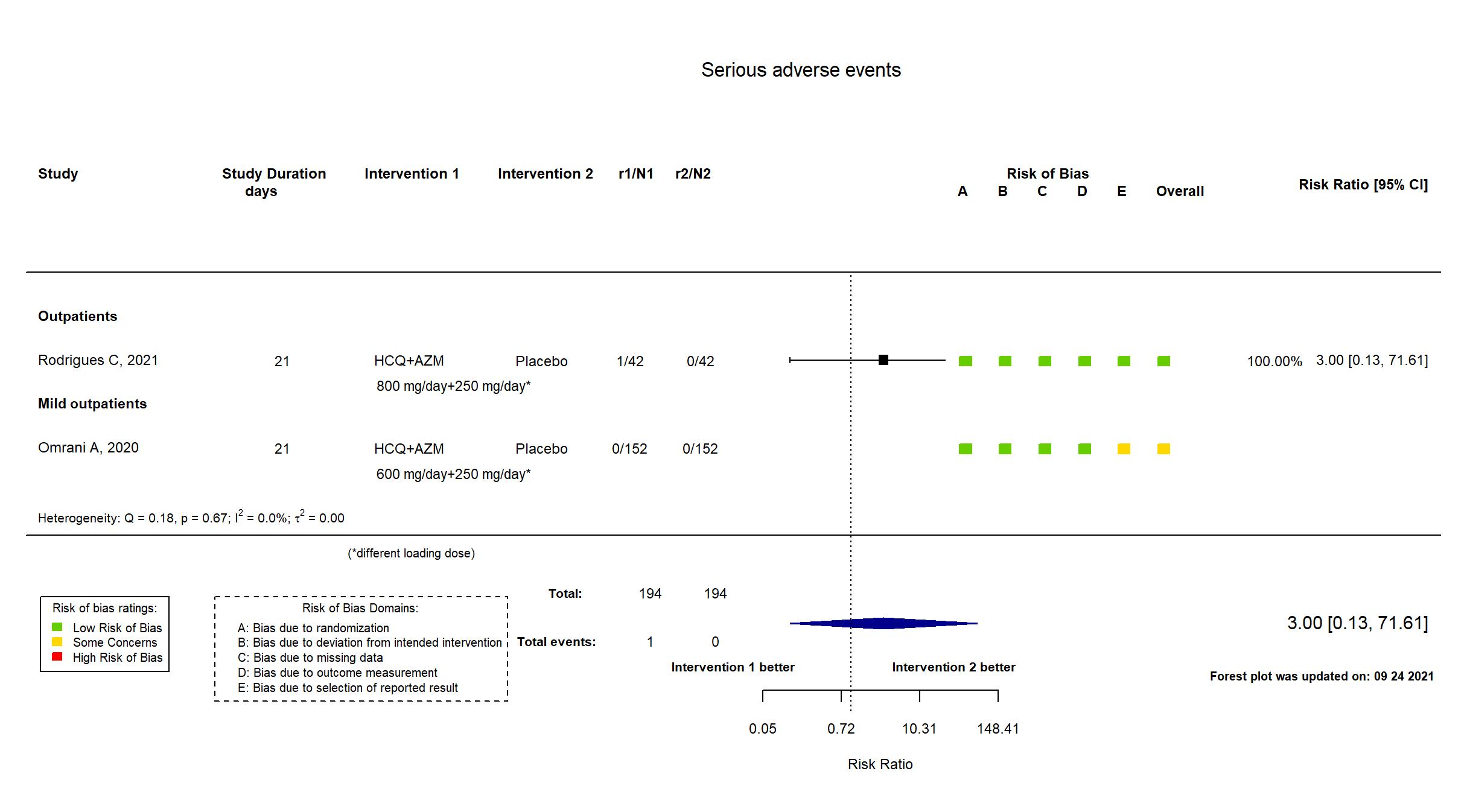




Trial NCT04349592
Publication Q-PROTECT - Omrani A, EClinicalMedicine (2020) (published paper)
Dates: 2020-04-13 to 2020-08-01
Funding: Public/non profit (Hamad Medical Corporation (government health service of the State of Qatar))
Conflict of interest: No
| Methods | |
| RCT Blinding: | |
| Location :
Multicenter / Qatar Follow-up duration (days): 21 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
HCQ+AZM Hydroxychloroquine: 200 mg orally 3 times a day for 7 days. Azithromycin: 500 mg orally on day 1 followed by 250 mg orally once a day for the next 4 days. Hydroxychloroquine 200 mg orally 3 times a day for 7 days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : HCQ+AZM=152 Hydroxychloroquine=152 Placebo=152 | |
| Characteristics of participants N= 456 Mean age : NR 449 males Severity : Mild: n= 456/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register Proportion of virologically cured (PCR-negative status) as assessed on day six [ Time Frame: Day 6 ] | |
| In the report Virologic cure (PCR-negative status) as assessed on day six. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan NR Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the trial registry and study protocol were used for data extraction and assessment of risk of bias. No statistical analysis plan was available. There were no substantive differences between the published article, the current trial registration and the study protocol in terms of procedures, population or treatments. The trial registry was changed near to end of recruitment to include outcomes not included in the original entry and the study protocol is not dated, but the data extracted are not affected. The study achieved its pre-stated sample size. Although open to both sexes and conducted in Qatar, the study population was overwhelmingly male and no Qataris were included, reflecting the country’s workforce. |
Trial RBR-95yjmq
Publication Rodrigues C, Int J Antimicrob Age (2021) (published paper)
Dates: 2020-04-12 to 2020-05-13
Funding: Private (Diagnósticos da América S.A. (Dasa), Ímpar Serviços Hospitalares S.A., and DNA Capital Foundation.)
Conflict of interest: No
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Single center / Brazil Follow-up duration (days): 21 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
HCQ+AZM HCQ: two 200 mg capsules twice a day for seven days + AZM: Initial dose: one 500 mg capsule on day 1 - Maintenance dose: one 250m mg capsule/day for 4 days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : HCQ+AZM=42 Placebo=42 | |
| Characteristics of participants N= 84 Mean age : NR 50 males Severity : Mild: n= 84/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register Time to negative viral load from the beginning of treatment and up to 9 days | |
| In the report Time (days) to viral clearance within a 9-day evaluation period following enrollment following the onset of symptoms and the study enrollment dates. | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published article, the retrospective study registry was used in data extraction and risk of bias assessment. Neither protocol nor statistical analysis plan was available There is no change from the trial registration in the intervention and control treatments. The primary outcome indicated in registry reflects the primary outcome reported in the paper. Some secondary outcomes in the registry (radiographic response, concomitant medication to manage symptoms, adverse events) were not reported or not fully reported. The study achieved the target sample size declared in the methods (n = 84), but not that in the registry (n = 108).
Risk of bias assessment was updated on May 15th 2022 after quality control. |