Hydroxychloroquine + Azithromycin vs Standard care/Placebo (RCT)
Hospitalized patients
FOREST PLOTS -2021-06-24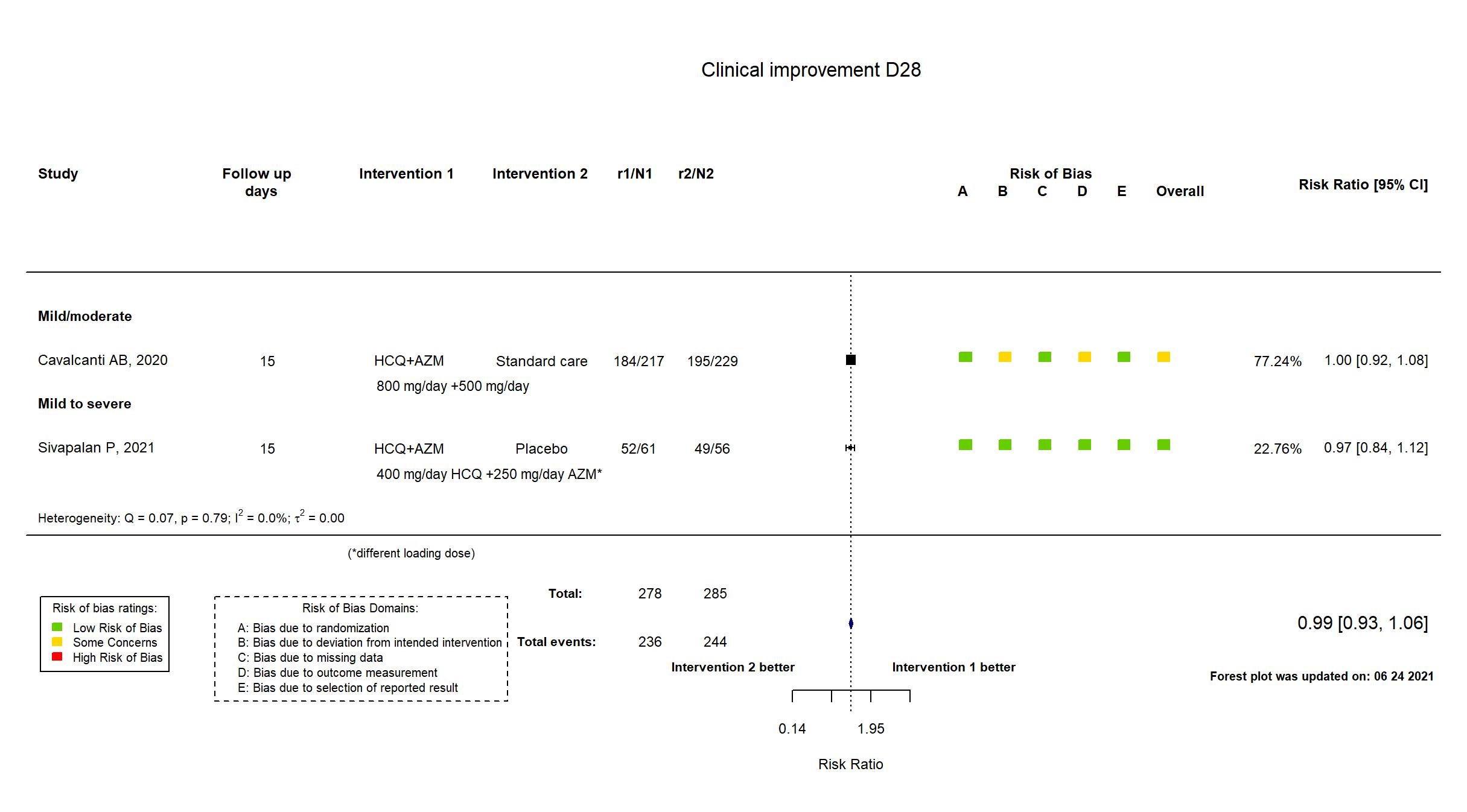
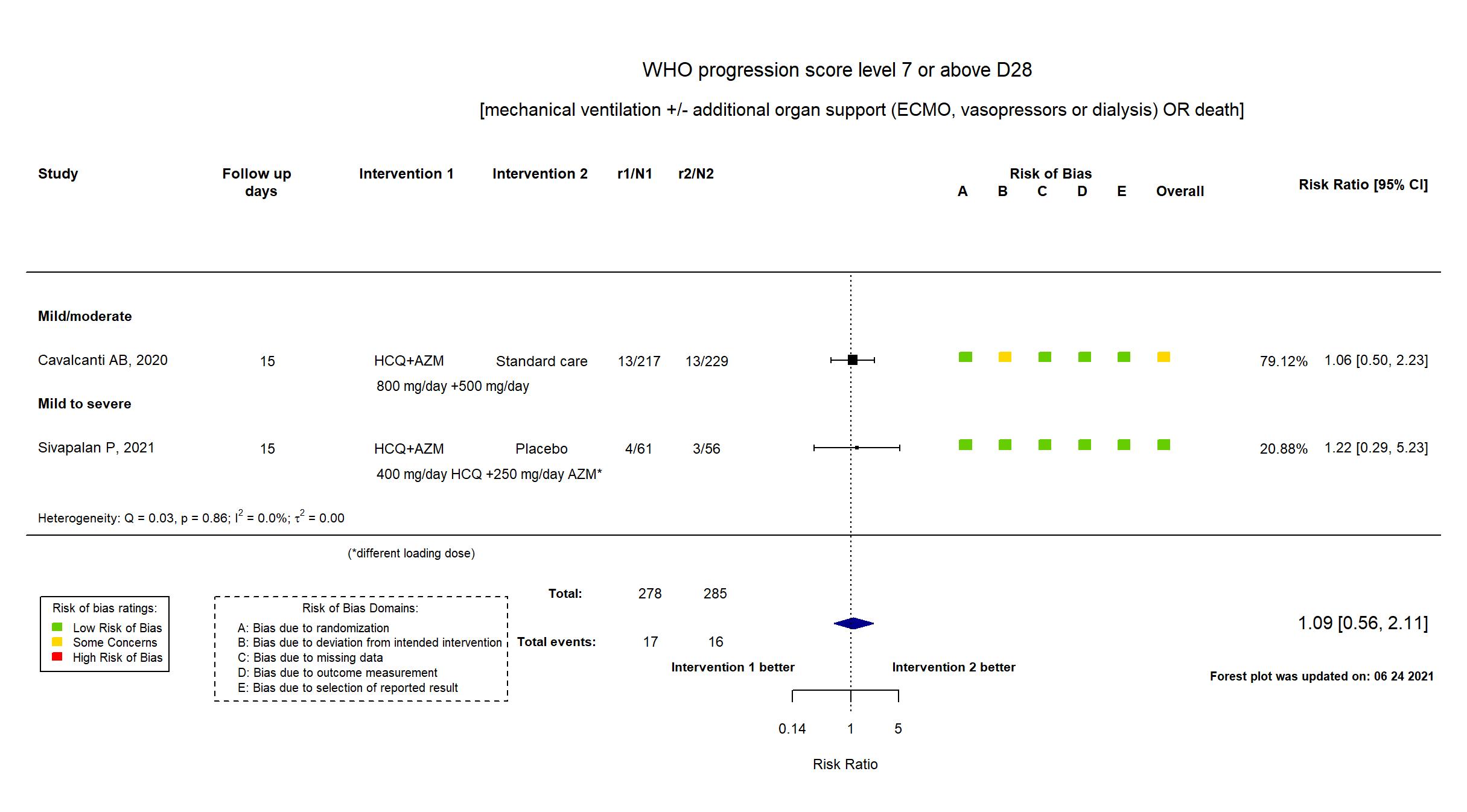
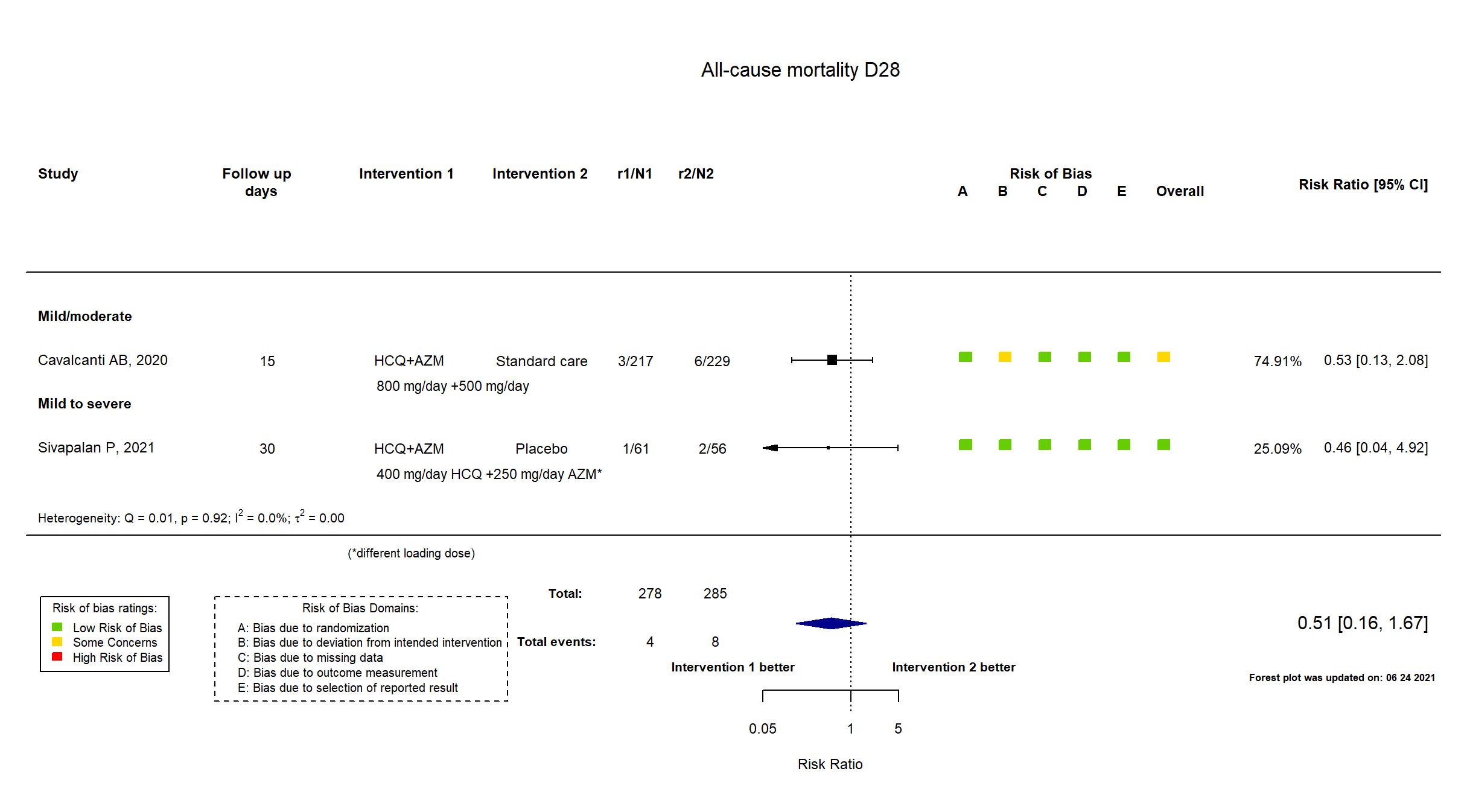
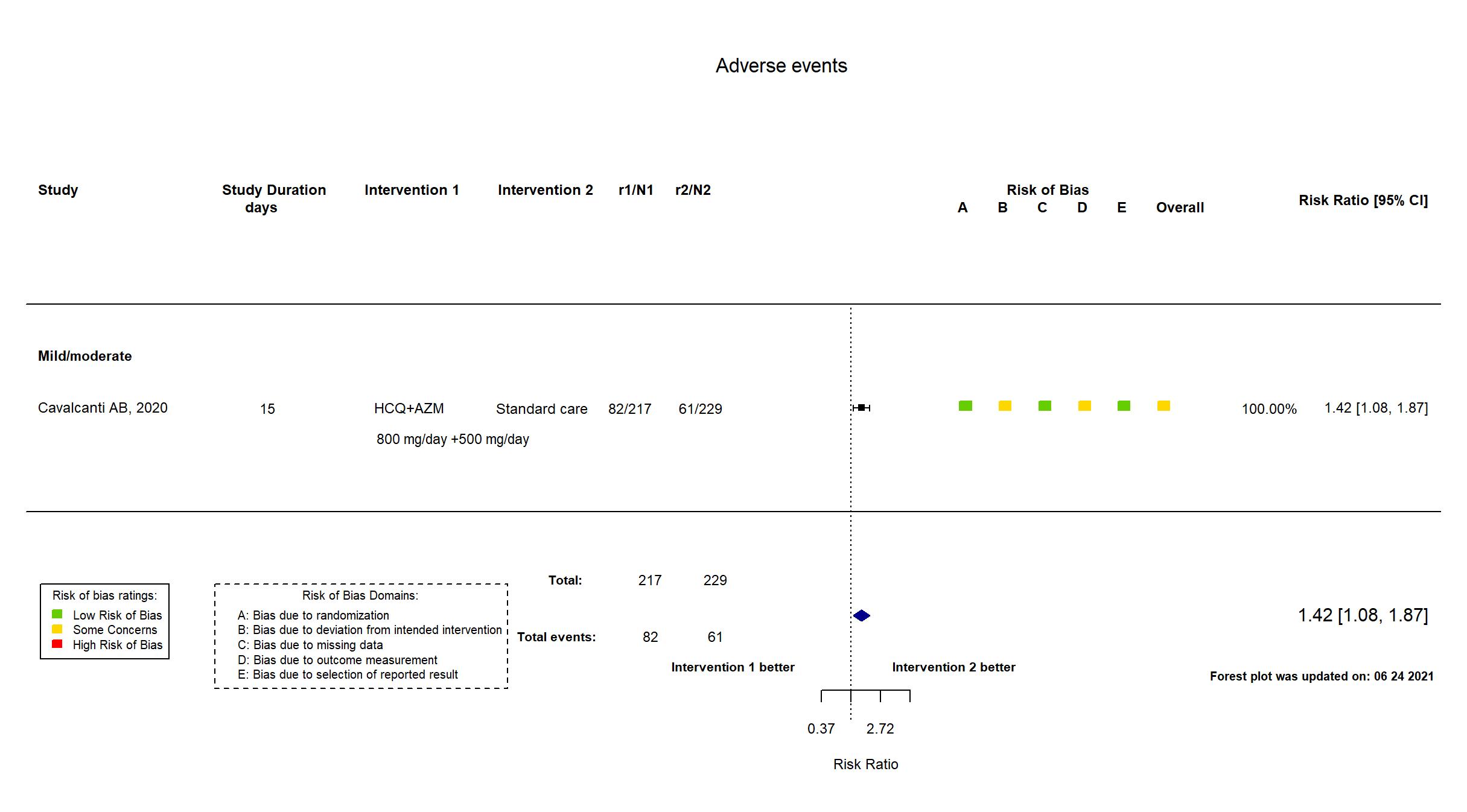






Trial NCT04322123
Publication Cavalcanti AB, N Engl J Med (2020) (published paper)
Dates: 3/29/2020 to 5/17/2020
Funding: Mixed (Hospitals and research institutes participating in Coalition Covid-19 Brazil; EMS Pharma )
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Brazil Follow-up duration (days): 15 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
HCQ+AZM Hydroxychloroquine: 400 mg orally or via nasogastric tube twice a day for 7 days Azithromycin: 500 mg IV or orally once a day for 7 days Hydroxychloroquine 400 mg orally or via nasogastric tube twice a day for 7 days |
|
| Control
Standard care | |
| Participants | |
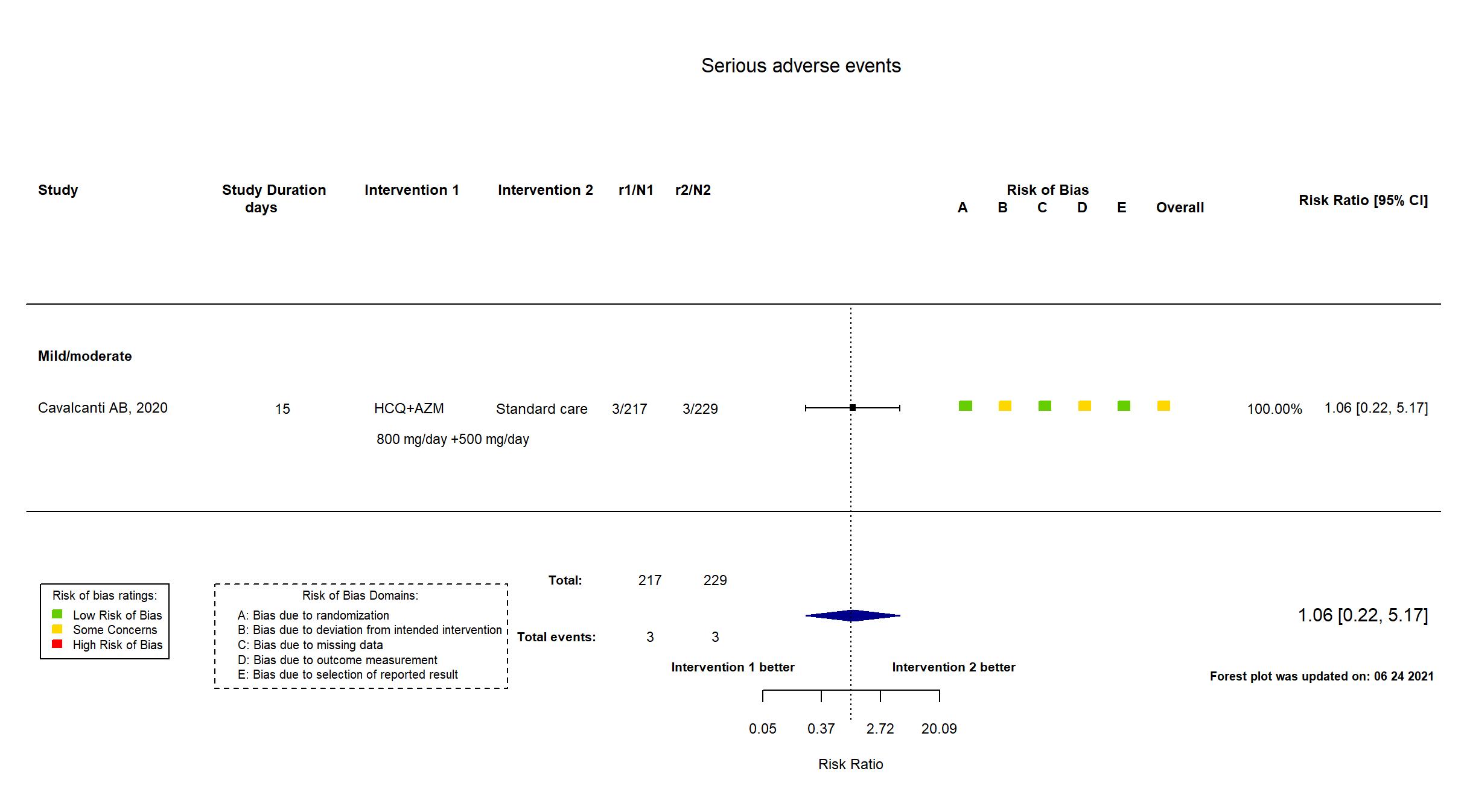
| Randomized participants : HCQ+AZM=217 Hydroxychloroquine=221 Standard care=229 | |
| Characteristics of participants N= 667 Mean age : NR 388 males Severity : Mild: n=387 / Moderate: n=278 / Severe: n=0 Critical: n=0 | |
| Primary outcome | |
| In the register Evaluation of the clinical status of patients on the 15th day after randomization defined by the Ordinal Scale of 7 points. | |
| In the report Clinical status at 15 days, evaluated with the use of a seven-level ordinal scale (with levels ranging from one to seven and higher scores indicating a worse condition) | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published report and supplementary file, the study registry, protocol and SAP were used in data extraction and risk of bias assessment. The study achieved the target sample size reported in the registry. There is no change from the trial registration in the intervention and control treatments. All outcomes from the registry are reported in the paper. Quote: "We had originally planned for the trial to include 630 patients, using the intention-to-treat analysis population, with a six-level ordinal outcome as the primary outcome, as described in the Supplementary Appendix. However, before the first interim analysis was conducted, we changed the primary-outcome assessment to the seven-level ordinal scale and the main analysis population from the intention-to-treat population to a modified intention-to-treat population that included only patients with a diagnosis of Covid-19 that had been confirmed by reverse-transcriptase- polymerase-chain-reaction (RT-PCR) testing (using the test available at each site)." Comment: Furthermore, safety analysis on a safety population of 4 arms was performed - hydrxychloroquine plus azithromycin (n=239), hydroxychloroquine only (n=199), azithromycin only (n=50)and neither hydroxychloroquine nor azithromycin (n=177). Data could not be accurately extracted from this safety population, therefore adverse events data from ITT population were used. |
Trial NCT04322396, EudraCT 2020-001198-55
Publication ProPAC-COVID - Sivapalan P, Eur Respir J (2021) (published paper)
Dates: 2020-04-06 to 2020-12-21
Funding: Mixed (The Novo Nordisk Foundation, Herlev and Gentofte Hospital, University Hospital of Copenhagen.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: quadruple blinding | |
| Location :
Multicenter / Denmark Follow-up duration (days): 30 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
HCQ+AZM HCQ: 200 mg orally twice a day for 15 days. AZM: 500 mg orally once a day for 3 days, then 250 mg orally once a day for 12 days. |
|
| Control
Placebo | |
| Participants | |
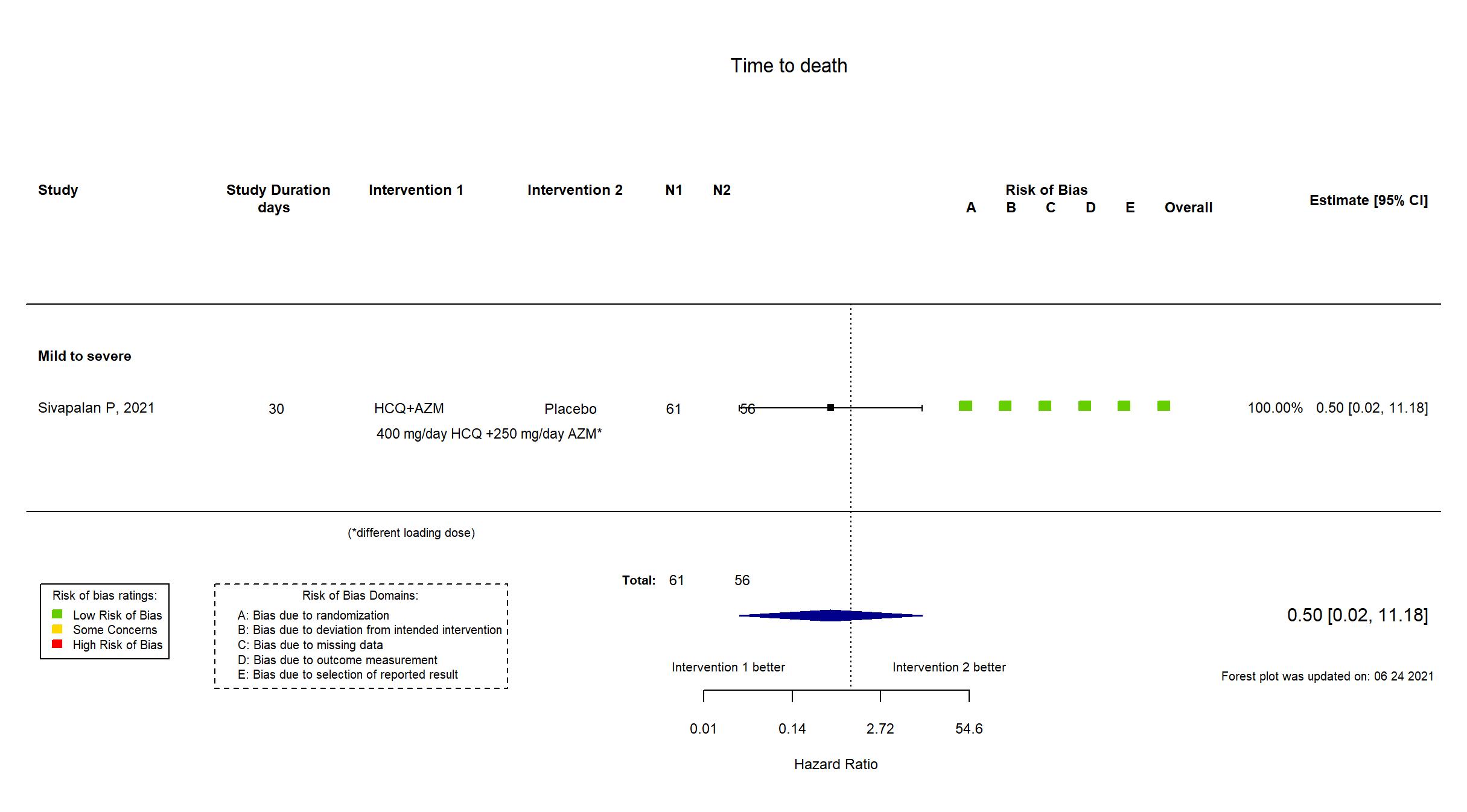
| Randomized participants : HCQ+AZM=61 Placebo=56 | |
| Characteristics of participants N= 117 Mean age : NR 65 males Severity : Mild: n=* / Moderate: n=* / Severe: n=27 Critical: n=0 | |
| Primary outcome | |
| In the register Days alive and discharged from hospital within 14 days | |
| In the report Days alive and out of hospital (DAOH) within 14 days from randomisation. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Low |
| General comment | In addition to the published article, the full and published study protocols and statistical analysis plan, prospective trial registries ( NCT04322396), EudraCT 2020-03-21) and supplementary appendices were used in data extraction and assessment of risk of bias. There were no substantive differences between the published article and the protocols or registries in population, procedures, interventions or outcomes. The registry and protocol primary outcome reflects the reported primary outcome. Recruitment was terminated at the first planned interim analysis after a recommendation from the data monitoring board based on pre-specified futility criteria, and therefore the study did not achieve its pre-specified target sample size. |