Azithromycin vs Standard care/Placebo (RCT)
Mild outpatients
FOREST PLOTS -2022-04-29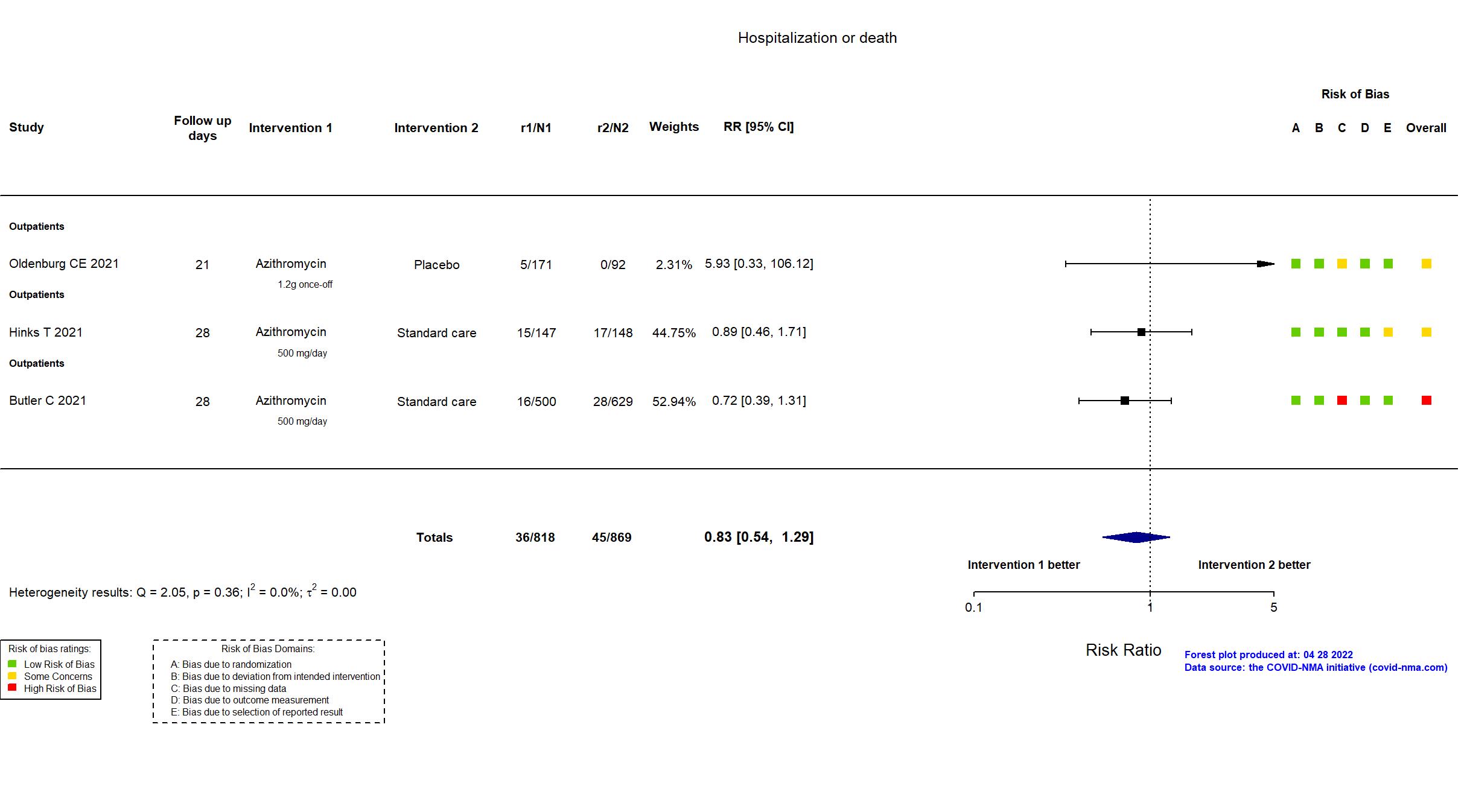





Trial ISRCTN86534580 ; EudraCT 2020-001209-22
Publication PRINCIPLE - Butler C, Lancet (2021) (published paper)
Dates: 2020-05-22 to 2020-11-30
Funding: Public/non profit (UK Research and Innovation and UK Department of Health and Social Care)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
* / UK Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Azithromycin 500 mg/day orally for 3 days |
|
| Control
Standard care | |
| Participants | |
| Randomized NR Analyzed 1129 participants Standard care=629 Azithromycin=500 | |
| Characteristics of participants N= 1129 Mean age : NR 486 males Severity : Mild: n= 1129/ Asymptomatic: n=0 | |
| Primary outcome | |
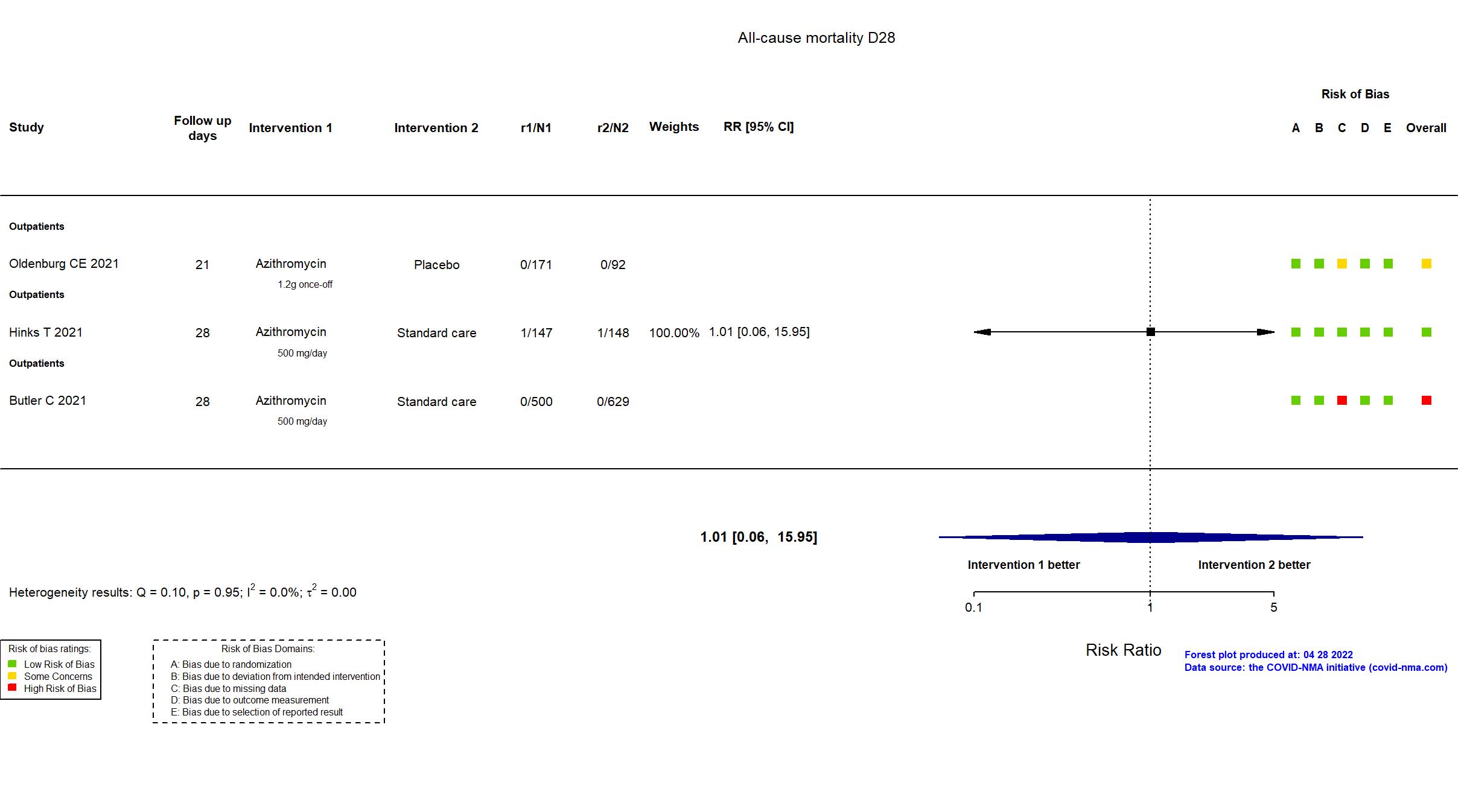
| In the register 1. Time taken to self-reported recovery, defined as the first instance that a participant reports feeling recovered from possible COVID-19 2. Hospitalisation and/or death | |
| In the report Coprimary endpoints: time to first self-reported recovery within 28 days from random assignment, with time to recovery defined as the first instance that a participant reported feeling recovered; and hospital admission or death within 28 days of random assignment. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
High |
| General comment |
In addition to the published article, the trial registries, study protocol, statistical analysis plan and supplementary materials were used in data extraction and assessment of risk of bias. This trial of azithromycin versus usual care for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course was part of the PRINCIPLE adaptive platform trial. There were no substantive differences between the article and the trial registries, study protocol and statistical analysis plan in population, procedures, interventions.
Quote: "The trial commenced with a single primary outcome: hospitalisation or death within 28 days. However, the proportions of people in the community requiring hospitalisation were much lower in the UK than seen in initial data from China, meaning a different outcome would be required to allow rapid evaluation of various interventions within reasonable sample sizes. The trial management and steering committees therefore recommended that the primary outcome be amended to include a measure of illness duration. This change was approved by the research ethics committee and the MHRA on September 16, 2020, and implemented before any interim analyses were done. Thus, the trial has coprimary endpoints, as follows: time to first self-reported recovery within 28 days from random assignment, with time to recovery defined as the first instance that a participant reported feeling recovered (ascertained by answering the question, “Do you feel recovered today? ie, symptoms associated with illness are no longer a problem. Yes/No”); and hospital admission or death within 28 days of random assignment." The published article reported comparisons of the azithromycin and usual care arms in both a primary analysis population (which included participants randomized to usual care before azithromycin arm was added to the platform trial) and a concurrent randomization analysis population (including only participants concurrently randomized to either azithromycin or usual care). It was determined that the latter concurrent randomization analysis population was most appropriate for the COVID NMA. The total number randomized, numbers missing from analysis and reasons were only reported in the usual care arm for the primary analysis population; therefore, the risk of bias due to missing data was unclear. Recruitment to the trial was terminated for futility. |
Trial NCT04381962
Publication ATOMIC2 - Hinks T, Lancet Respir Med (2021) (published paper)
Dates: 2020-06-03 to 2021-01-29
Funding: Mixed (NIHR Oxford BRC, University of Oxford and Pfizer Inc)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / UK Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Azithromycin 500 mg orally once daily for 14 days |
|
| Control
Standard care | |
| Participants | |
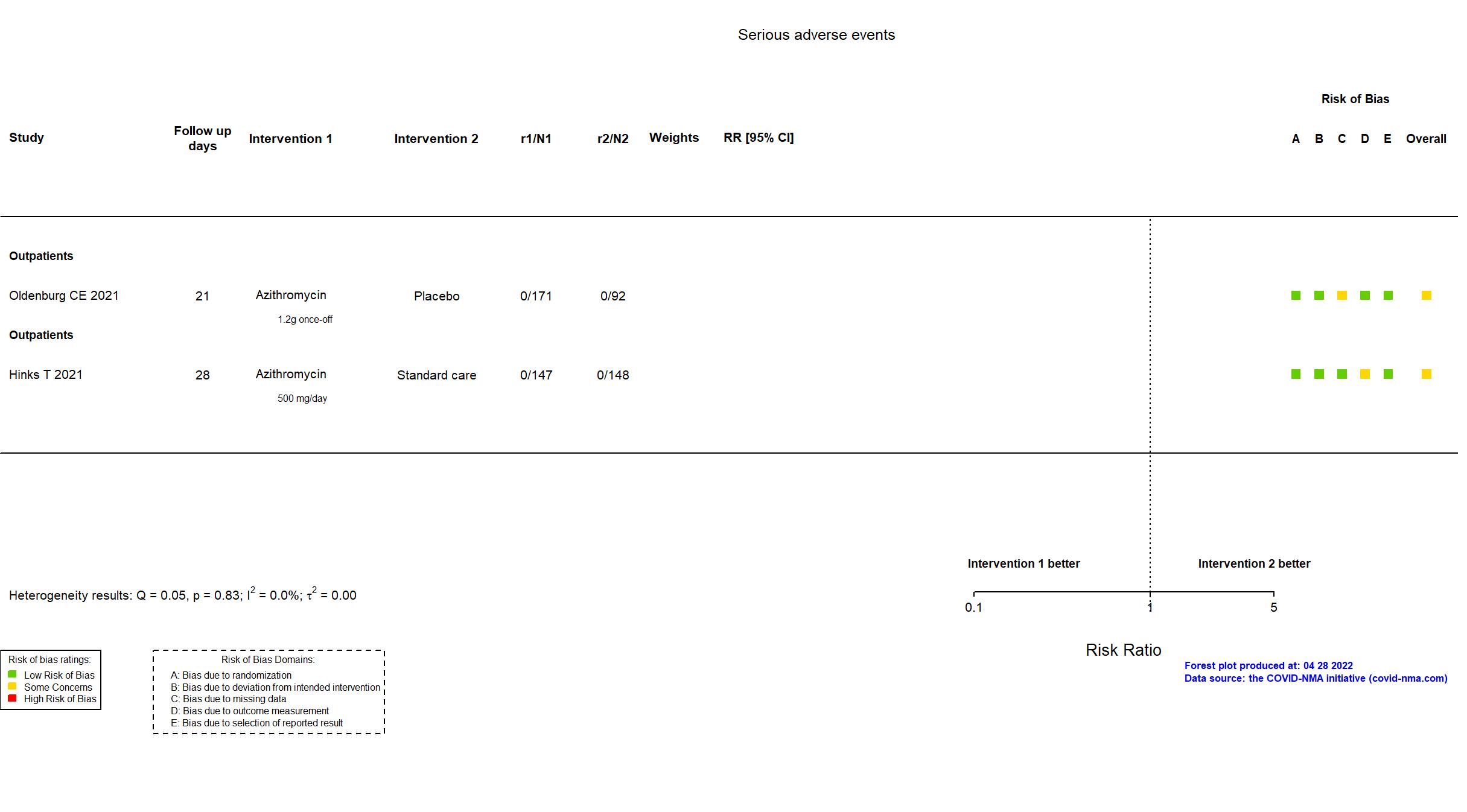
| Randomized participants : Azithromycin=147 Standard care=148 | |
| Characteristics of participants N= 295 Mean age : NR 152 males Severity : Mild: n= 293/ Asymptomatic: n=0 | |
| Primary outcome | |
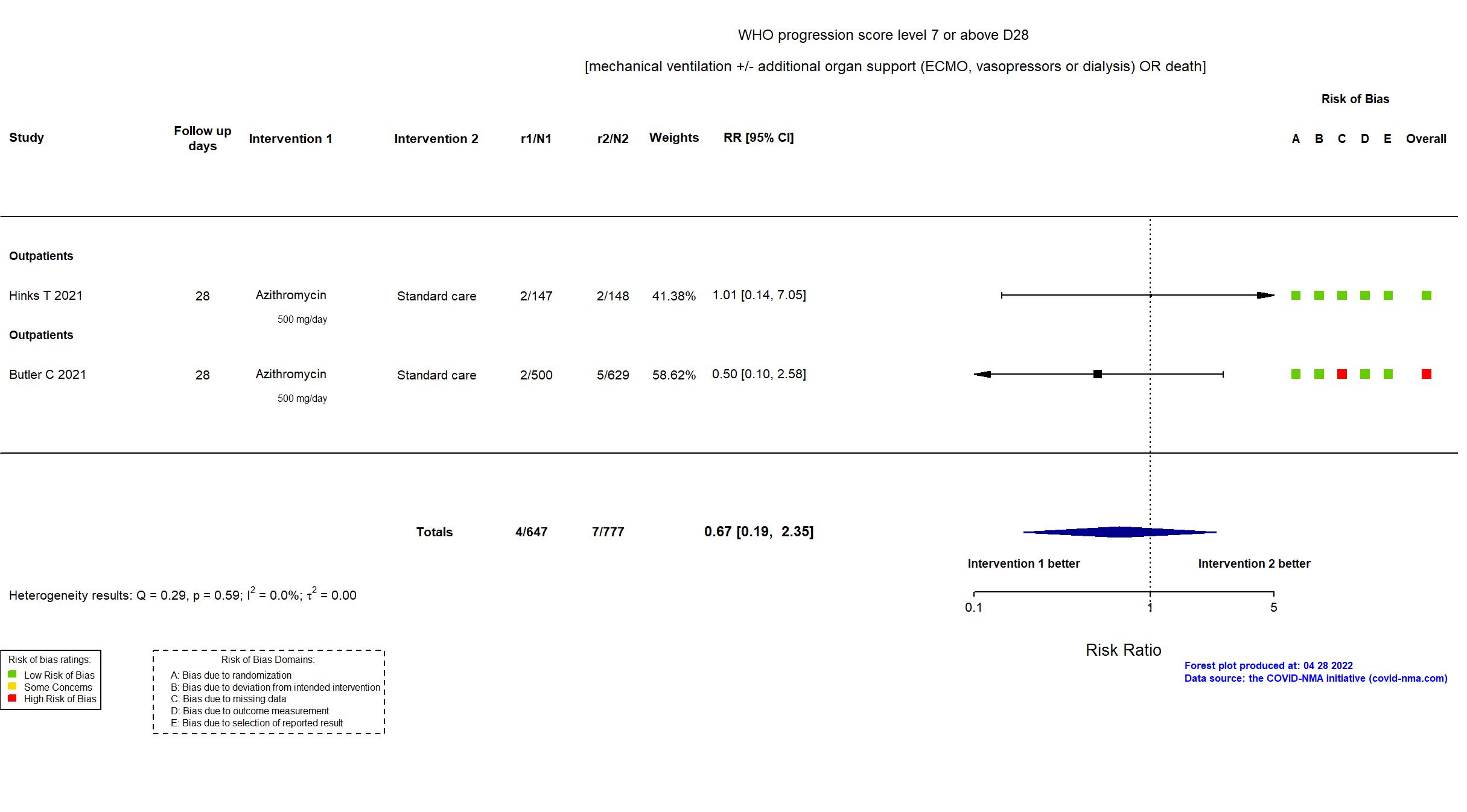
| In the register Proportion progressing to respiratory failure or death (all clinically-diagnosed participants) [ Time Frame: Determined at day 28 from randomisation.] | |
| In the report Proportion of participants with hospital admission or death from any cause within 28 days from randomisation | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the pre-print article, the trial registry, protocol and statistical analysis plan were used in data extraction and assessment of risk of bias. There were no differences between the article and the registry or protocol in population, procedures and interventions. The primary outcome in the pre-print article (proportion of participants with hospital admission or death from any cause) was changed on advice from the data safety monitoring committee and with ethical approval from the original primary outcome in the registry and protocols (proportion progressing to hospitalization with respiratory failure or death) due to lack of events at interim analysis. The sample size in the registry was recalculated in accordance WHO recommendations that a pilot phase with 100 patients would be sufficient to inform follow-on clinical research and reduced from 800 to 291. Some outcomes reported in the registry are not reported in the paper (mechanistic analysis of blood and nasal biomarkers).
This study was updated on July 28th, 2021 with data from contact with authors and publication. |
Trial NCT04332107
Publication Oldenburg CE, JAMA (2021) (published paper)
Dates: 2020-05-22 to 2021-03-16
Funding: Mixed (Bill & Melinda Gates Foundation, intervention drugs were donated by Pfizer Inc.)
Conflict of interest: No
| Methods | |
| RCT Blinding: quadruple blinding | |
| Location :
Multicenter / USA Follow-up duration (days): 21 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Azithromycin 1.2 g orally once-off |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Azithromycin=171 Placebo=92 | |
| Characteristics of participants N= 263 Mean age : NR 86 males Severity : Mild: n= 245/ Asymptomatic: n=18 | |
| Primary outcome | |
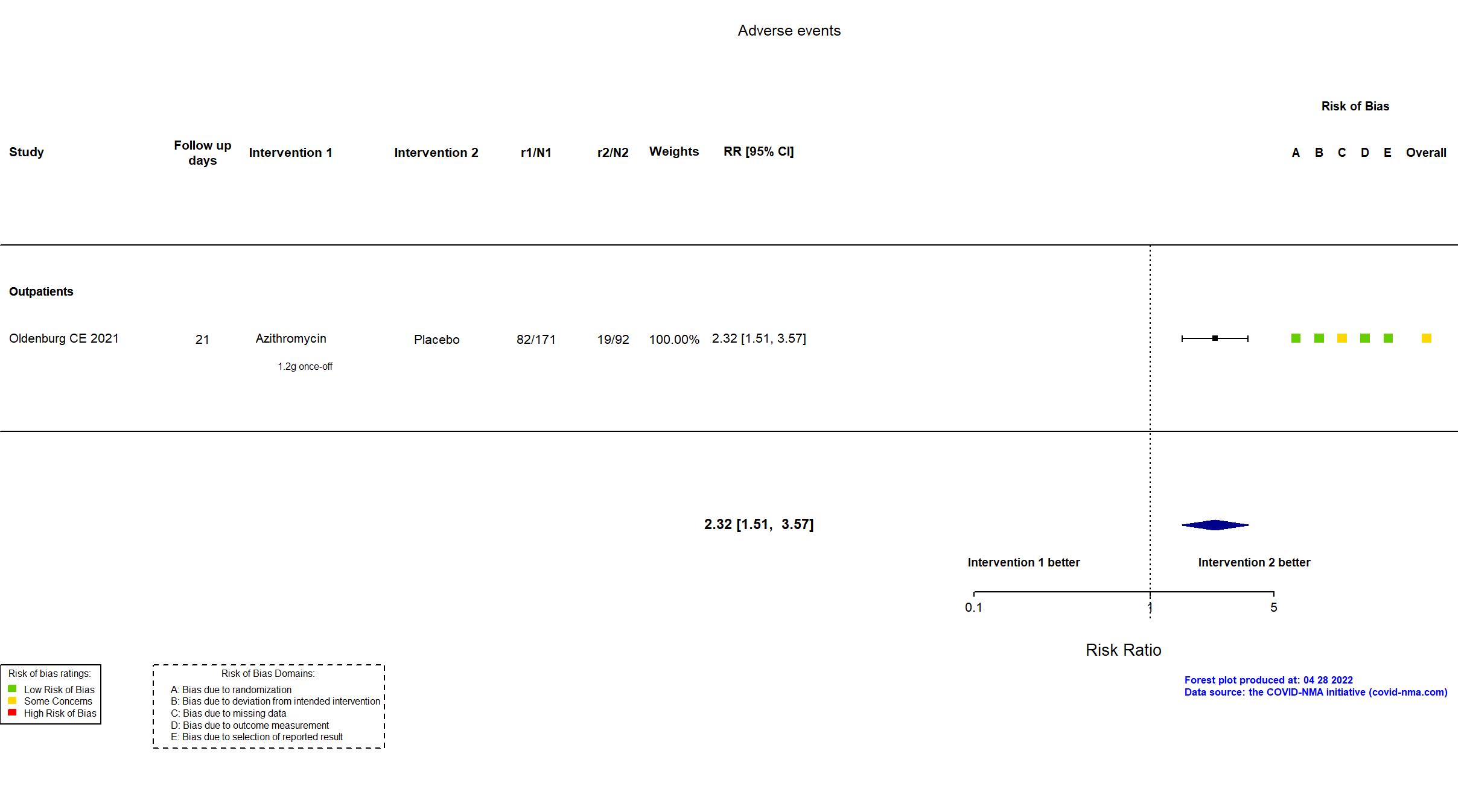
| In the register Binary assessment of whether the participant is experiencing any symptoms at Day 14 (yes or no) | |
| In the report Self-reported absence of COVID-19 symptoms at day 14 | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the study registry, protocol, and statistical analysis plan were used in data extraction and risk of bias assessment. The study was terminated at interim analysis by the data monitoring board due to futility, consequently, planned sample size was not reached. There is no change from the trial registration in the intervention and control treatments. The primary outcome changed from hospitalization to absence of symptoms during the course of the trial, with consultation and approval by the data monitoring board. |
Trial NCT04622891
Publication Rashad A, Scientific Reports (2021) (published paper)
Funding: Public/non profit (South Valley University, faculty of Medicine, Qena83523, Egypt)
Conflict of interest: No
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Single center / Egypt Follow-up duration (days): 15 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Azithromycin 500 mg orally once a day for 7 days Clarithromycin 500 mg orally twice a day for 7 days |
|
| Control
Standard care | |
| Participants | |
| Randomized NR Analyzed 305 participants Azithromycin=107 Clarithromycin=99 Standard care=99 | |
| Characteristics of participants N= 305 Mean age : NR 214 males Severity : Mild: n= 305/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register Time to fever control [ Time Frame: 15 days ] time to complete resolution of fever | |
| In the report NR | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
* |
| General comment |
This study is pending contact with authors. No review-specific outcomes reported/extracted.
The journal and pre-print articles was used in data extraction and risk of bias assessment. No protocol or statistical analysis plan was available. Consequently, there was no information on whether the trial was conducted and analyzed as planned. There was a large proportion of missing outcome data. The primary outcome, exclusion criteria and the proportion of participants randomized per arm were not reported. No outcome data were reported in a manner that allowed for extraction. This trial was updated on September 27th, 2021 with data from the peer-reviewed journal publication. |