Azithromycin vs Standard care/Placebo (RCT)
Hospitalized patients
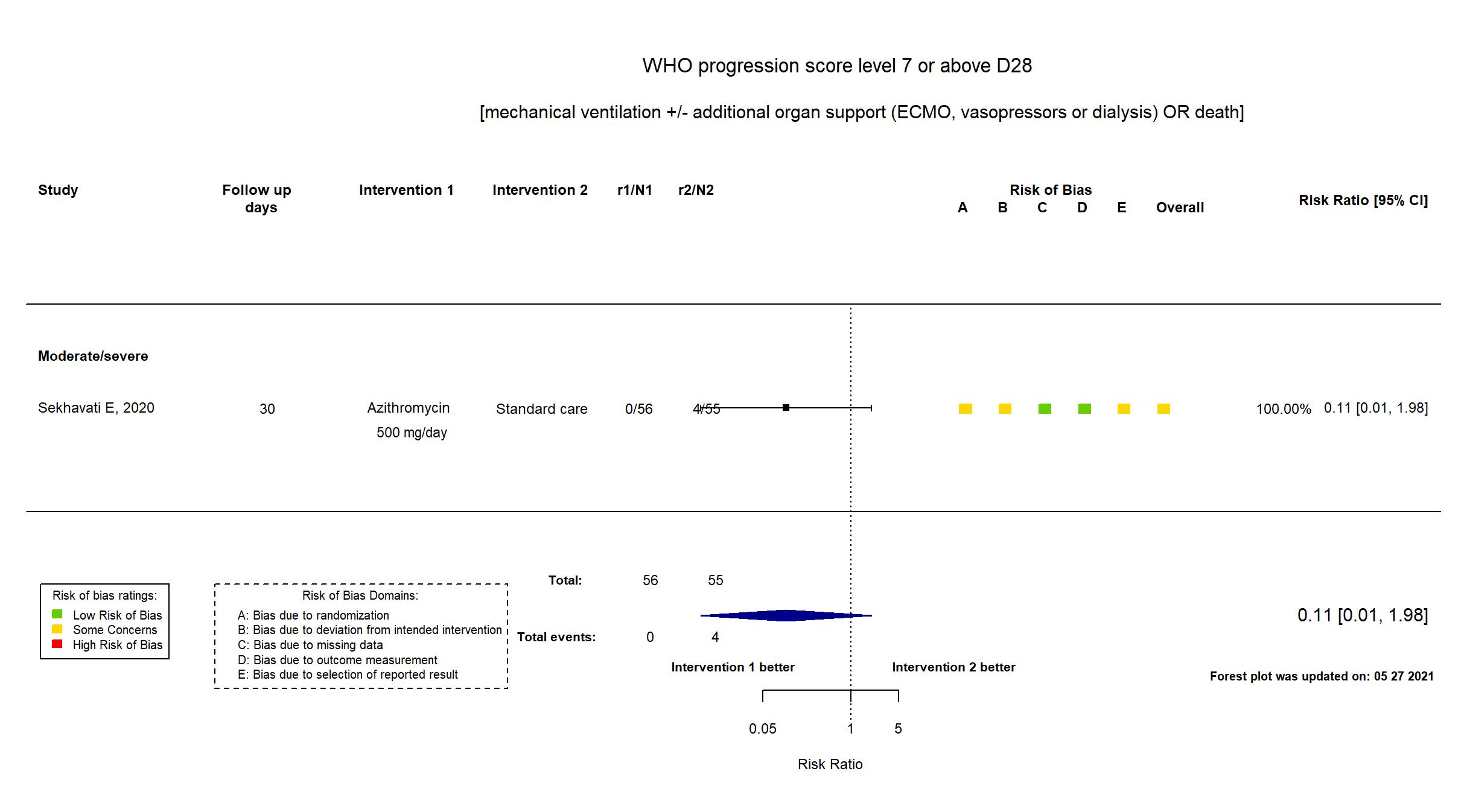
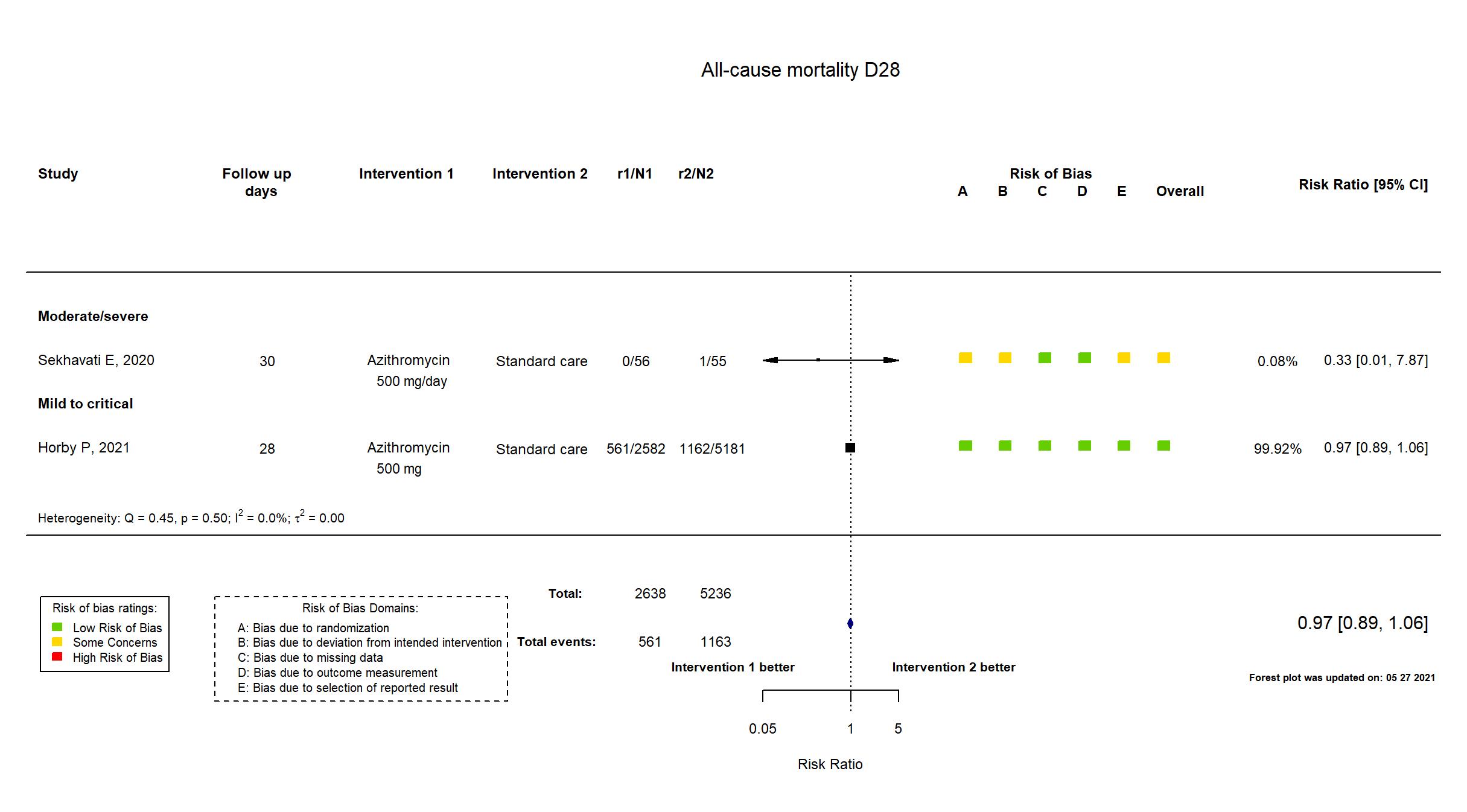
FOREST PLOTS -2021-05-27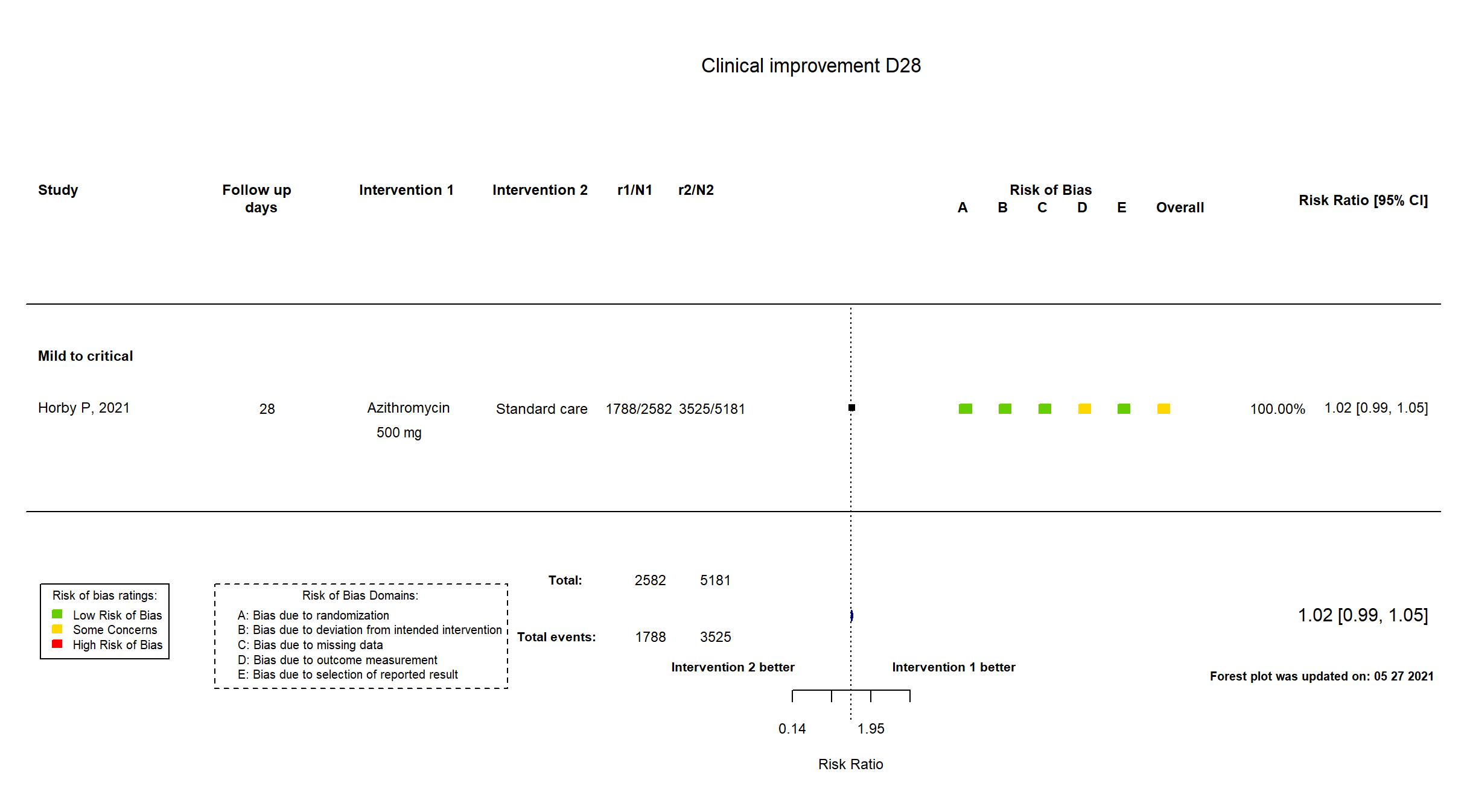



Trial NCT04381936 ; ISRCTN (50189673)
Publication RECOVERY (AZM) - Horby P, Lancet (2021) (published paper)
Dates: 2020-04-07 to 2020-11-27
Funding: Mixed (UK Medical Research Council, National Institute for Health Research, Wellcome, Bill and Melinda Gates Foundation, Dept for Int'l Development, Health Data Research UK. Drugs were donated by Abbvie, Roche & Regeneron.)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / UK Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Azithromycin 500 mg once a day orally, IV infusion or by nasogastric tube for 10 days or until discharge (if sooner) |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Standard care=5181 Azithromycin=2582 | |
| Characteristics of participants N= 7763 Mean age : NR 4819 males Severity : Mild: n=* / Moderate: n=* / Severe: n=* Critical: n=452 | |
| Primary outcome | |
| In the register All-cause mortality [ Time Frame: Within 28 days after randomisation ] | |
| In the report All-cause mortality | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to all available versions of the pre-print article, the study registries, statistical analysis plan, protocol and supplementary appendix were used in data extraction and risk of bias assessment.
The RECOVERY trial is an investigator-initiated, individually randomized, controlled, open-label, adaptive platform trial to evaluate the effects of potential treatments in patients hospitalized with COVID-19. The trial was conducted at 176 National Health Service (NHS) hospital organizations in the United Kingdom. Interim analysis of a trial in which recruitment has been completed but follow up is ongoing. There were no substantive differences in study procedures, population, interventions and outcomes between the pre-print article and the trial registries, study protocol and statistical analysis plan. The study achieved its pre-stated sample size. Quote: "The 2059 patients (27%) who had not been followed for 28 days and were not known to have died by the time of the data cut for this preliminary analysis (30 November 2020) were either censored on 30 November 2020 or, if they had already been discharged alive, were right-censored for mortality at day 29 (that is, in the absence of any information to the contrary they were assumed to have survived 28 days). [Note: This censoring rule will not be necessary for the final report.] We used similar methods to analyse time to hospital discharge and successful cessation of invasive mechanical ventilation, with patients who died in hospital right-censored on day 29." "Initially, recruitment was limited to patients aged at least 18 years but from 9 May 2020, the age limit was removed." "For some patients, azithromycin was unavailable at the hospital at the time of enrolment or if a macrolide antibiotic was considered by the managing physician to be either definitely indicated or definitely contraindicated. These patients were excluded from the randomised comparison between azithromycin and usual care." This study was updated on March 18th, 2021 after publication of the study report. |
Trial IRCT20200415047092N1
Publication Sekhavati E, Int J Antimicrob Age (2020) (published paper)
Dates: 2020-04-24 to 2020-05-08
Funding: Public/non profit (Tehran University of Medical Sciences research centre)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / Iran (Islamic Republic of) Follow-up duration (days): 30 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Azithromycin 500 mg orally once a day for 5 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Azithromycin=56 Standard care=55 | |
| Characteristics of participants N= 111 Mean age : NR 51 males Severity : Mild: n=0 / Moderate: n=* / Severe: n=* Critical: n=0 | |
| Primary outcome | |
| In the register Peripheral capillary oxygen saturation (daily during admission), Admission duration; Fever; Need to ICU admission | |
| In the report Decrease in mortality, duration of hospitalisation and need for intensive care unit (ICU) admission | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published study report, the trial registry was used in data extraction and assessment of risk of bias. Neither study protocol nor statistical analysis plane was available. The study achieved its target sample size. Due to cardiac risks associated with the concomitant use of Azithromycin and Hydroxychloroquine, patients with elevated risk for ventricular arrhythmia were excluded from the combined treatment arm, but not from the standard treatment arm. There were some differences between registry and published report in inclusion criteria and outcomes. There is no change from the trial registration in the intervention and control treatments. |