Losartan vs Standard care/Placebo (RCT)
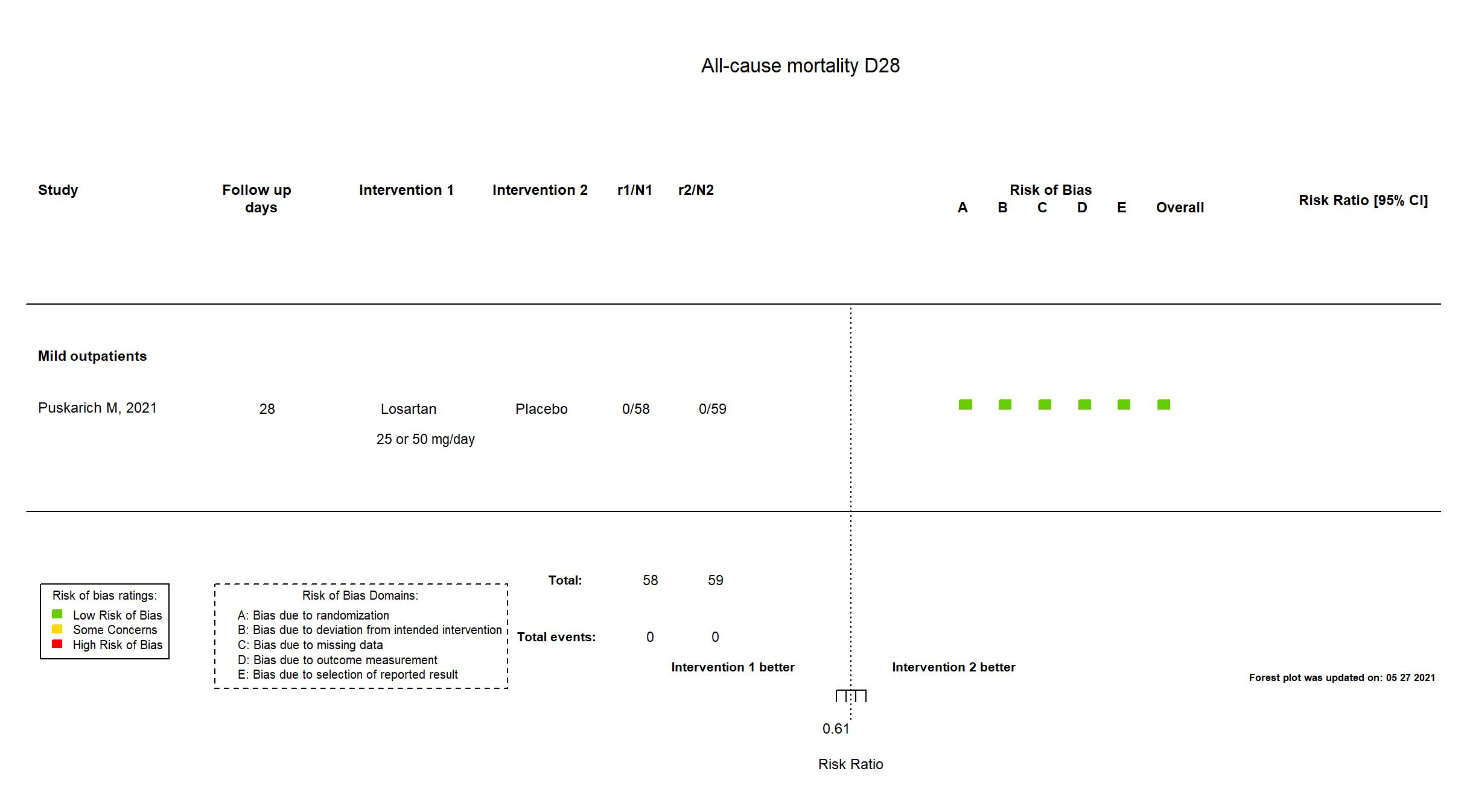
Mild outpatients
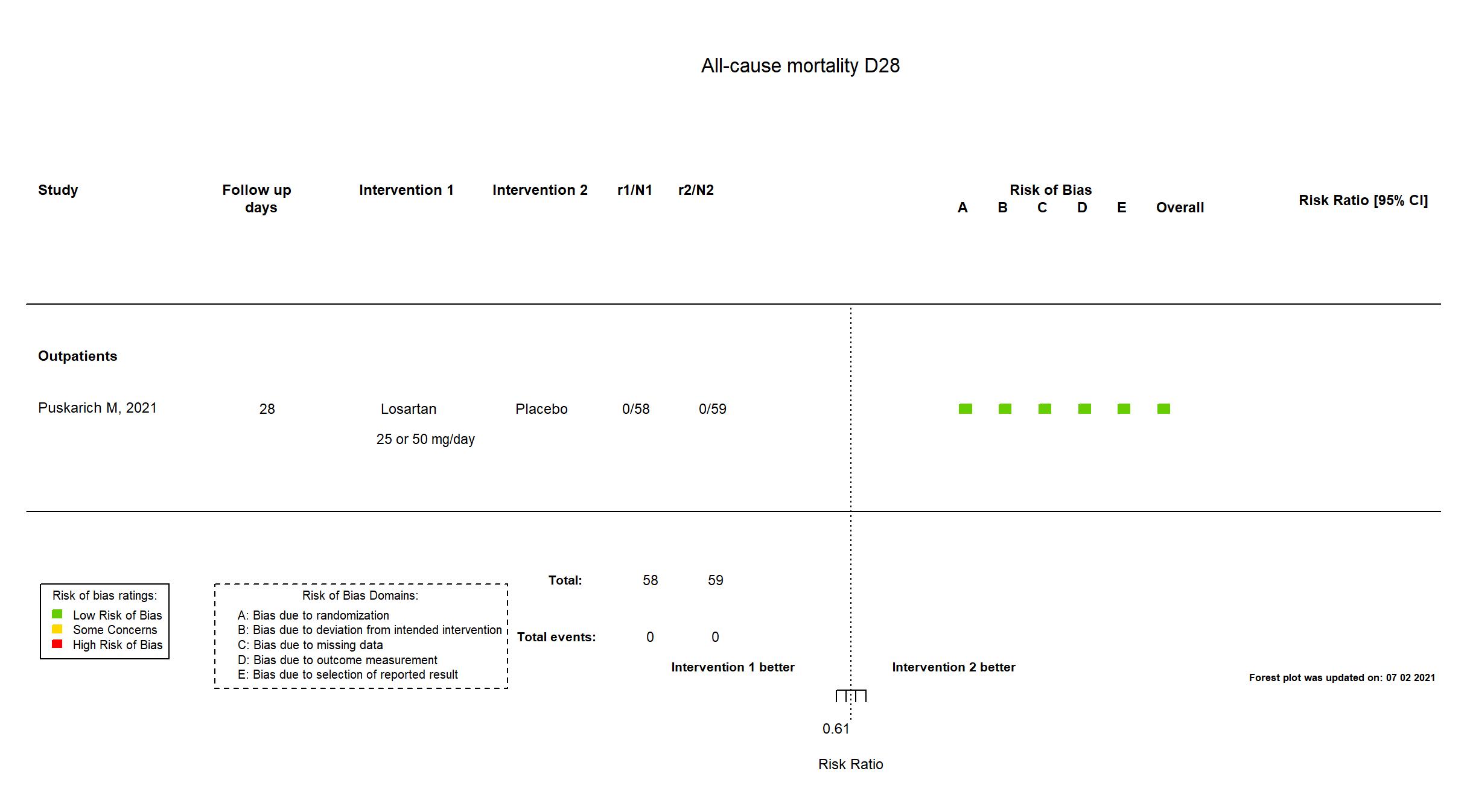
FOREST PLOTS -2021-07-02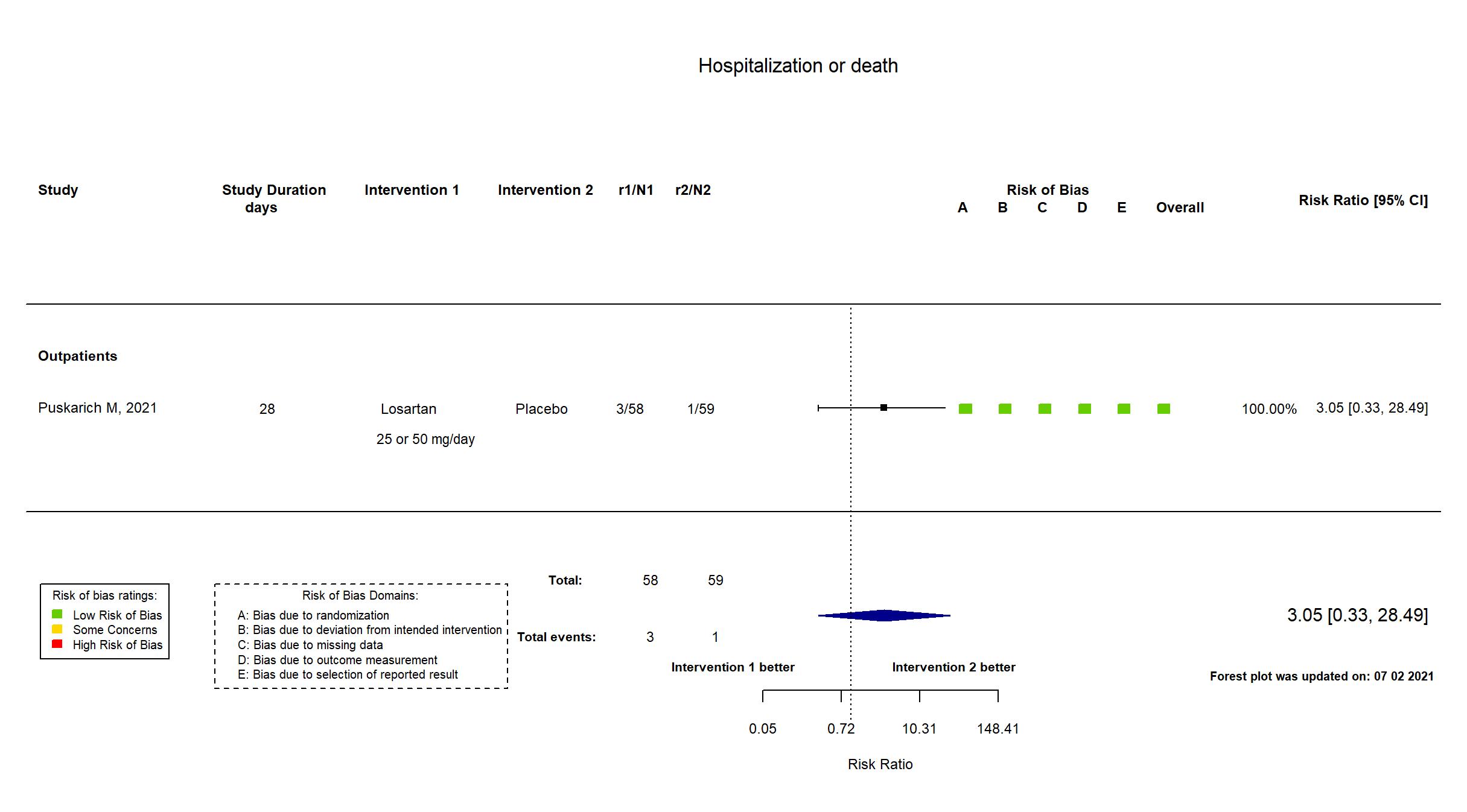



Trial NCT04311177
Publication Puskarich M, EClinicalMedicine (2021) (published paper)
Dates: 2020-04-09 to 2020-11-30
Funding: Public/non profit (Minnesota Partnership for Biotechnology and Medical Genomics)
Conflict of interest: No
| Methods | |
| RCT Blinding: Participants, outcome assessor and health care pro | |
| Location :
Multicenter / USA Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Losartan 25 mg orally twice daily (eGFR >60 mL/min/1.73 m2) or once daily (eGFR 30-60 mL/min/1.73 m2) for 10 days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Losartan =58 Placebo=59 | |
| Characteristics of participants N= 117 Mean age : NR 59 males Severity : Mild: n= 117/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register Hospital Admission [ Time Frame: 15 days ] | |
| In the report All-cause hospitalization within 15 days | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Low |
| General comment |
In addition to the pre-print article, the prospective trial registry was used in data extraction and risk of bias assessment. The trial was terminated early by the investigators due to low event rates, logistical challenges, and concern of more equitable allocation of potential study participants into larger national outpatient trial efforts more likely to identify an effective therapy. Consequently, the trial did not achieve the target sample size specified in the trial registry and several outcomes listed in the registry were not reported in the pre-print. However, the primary outcome (hospital admission) is the same in registry and report, and so are participant eligibility criteria and intervention- and control treatments.
This trial was updated on July 1st, 2021 with data from the published journal report. |