Losartan vs Standard care/Placebo (RCT)
Hospitalized patients
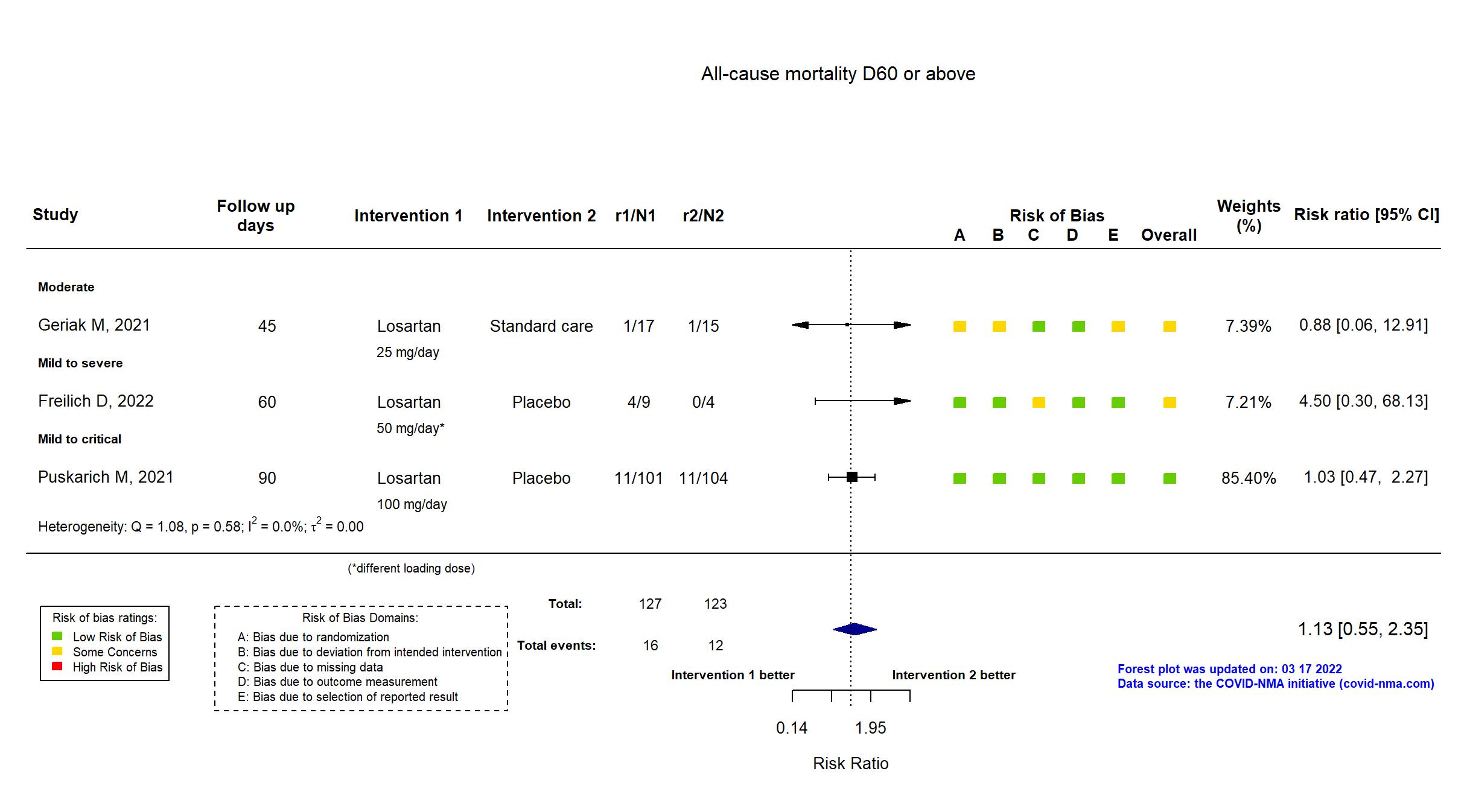
FOREST PLOTS -2022-03-17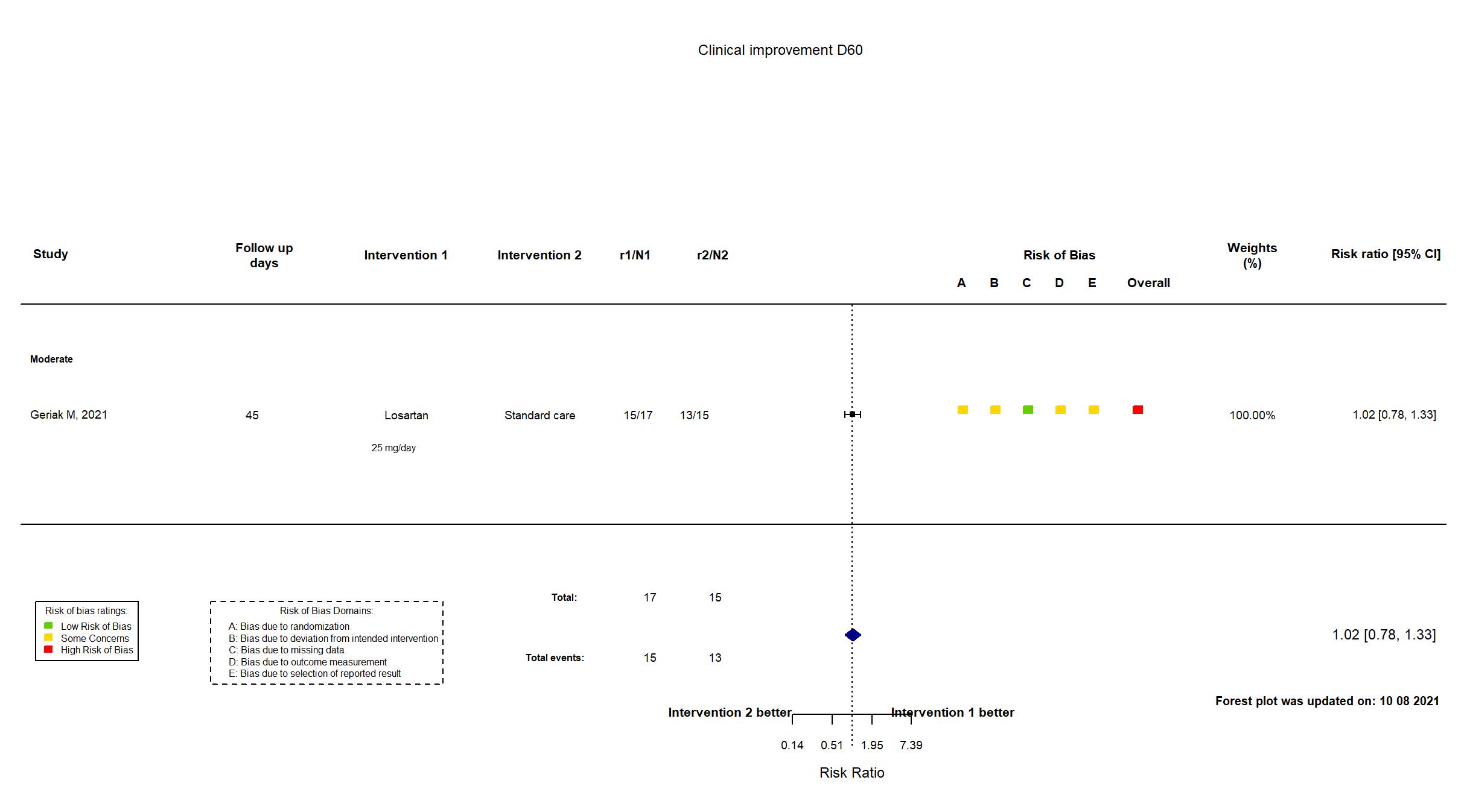




Trial NCT04328012
Publication COVID MED - Freilich D, medRxiv (2022) (preprint)
Dates: 2021-04-06 to 2021-05-27
Funding: Public/non profit (Bassett Research Institute and Bassett Medical Center’s Department of Internal Medicine)
Conflict of interest: No
| Methods | |
| RCT Blinding: quadruple blinding | |
| Location :
Single center / USA Follow-up duration (days): 60 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Losartan Initially 25 mg orally twice daily; changed to 25 mg orally once daily (after other treatment arms stopped) for up to 14 days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Losartan =9 Placebo=4 | |
| Characteristics of participants N= 13 Mean age : NR 8 males Severity : Mild: n=* / Moderate: n=* / Severe: n=* Critical: n=0 | |
| Primary outcome | |
| In the register National Institute of Allergy and Infectious Diseases COVID-19 Ordinal Severity Scale (NCOSS) [Time Frame: 60 days] | |
| In the report Improvement in the 7-point COVID-19 Ordinal Scale Score (COSS) | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | “In addition to the preprint article, the study registry was used in data extraction and risk of bias assessment. Neither the protocol nor the statistical analysis plan was available. The sample size is very low; the study stopped early due to low enrollment and did not reach the target sample size (n=100)." |
Trial NCT04340557
Publication Geriak M, Infect Dis Ther (2021) (published paper)
Dates: 2020-03-30 to 2020-07-04
Funding: Public/non profit ( Sharp Healthcare Foundation (San Diego, CA, USA))
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / USA Follow-up duration (days): 45 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Losartan 12.5 mg twice daily for up to 10 days. Dose could be escalated on judgement of treating physician. |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Losartan =17 Standard care=15 | |
| Characteristics of participants N= 32 Mean age : NR 19 males Severity : Mild: n=0 / Moderate: n=32 / Severe: n=0 Critical: n=0 | |
| Primary outcome | |
| In the register Mechanical ventilation [ Time Frame: from date of patient admission to date of patient discharge or date of death, whichever came first, assessed up to 45 days ] | |
| In the report Receipt of mechanical ventilation or death before receiving ventilation (a composite of either receiving ventilation or the patient status changed to a do not resuscitate/do not intubate resulting in progressive respiratory failure and death). | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
High |
| General comment | In addition to the published article, the trial registry was used in data extraction and assessment of risk of bias. Neither protocol nor statistical analysis plan was available. The authors report in the published article that the trial “was registered on clinicaltrials.gov (March 27, 2020; NCT04340557), inferring prospective registration. Clinicaltrials.gov reports the registration was first submitted on April 6, 2020, a week after start of recruitment. There were no differences between the registry and published article in population, procedures or interventions. There were some differences in the outcomes reported (receipt of mechanical ventilation or death before receiving ventilation, discharge without progression to ICU, ICU transfer, in-hospital mortality and length of hospital stay) and those in the registry (number requiring transfer into ICU for mechanical ventilation, number transferred from non-ICU bed to an ICU bed, number of days requiring oxygen therapy). Adverse events were not reported except for discontinuation due to tolerability/safety. Mortality was reported as in-hospital mortality. The study did not achieve its estimated sample size (n = 200; n = 32 randomized) and appears to have been terminated: “study enrollment dropped off consider- ably because of a rise in competing clinical trials”. The study was assessed to be at a high risk of bias due to some concerns in four of five domains. |
Trial NCT04312009
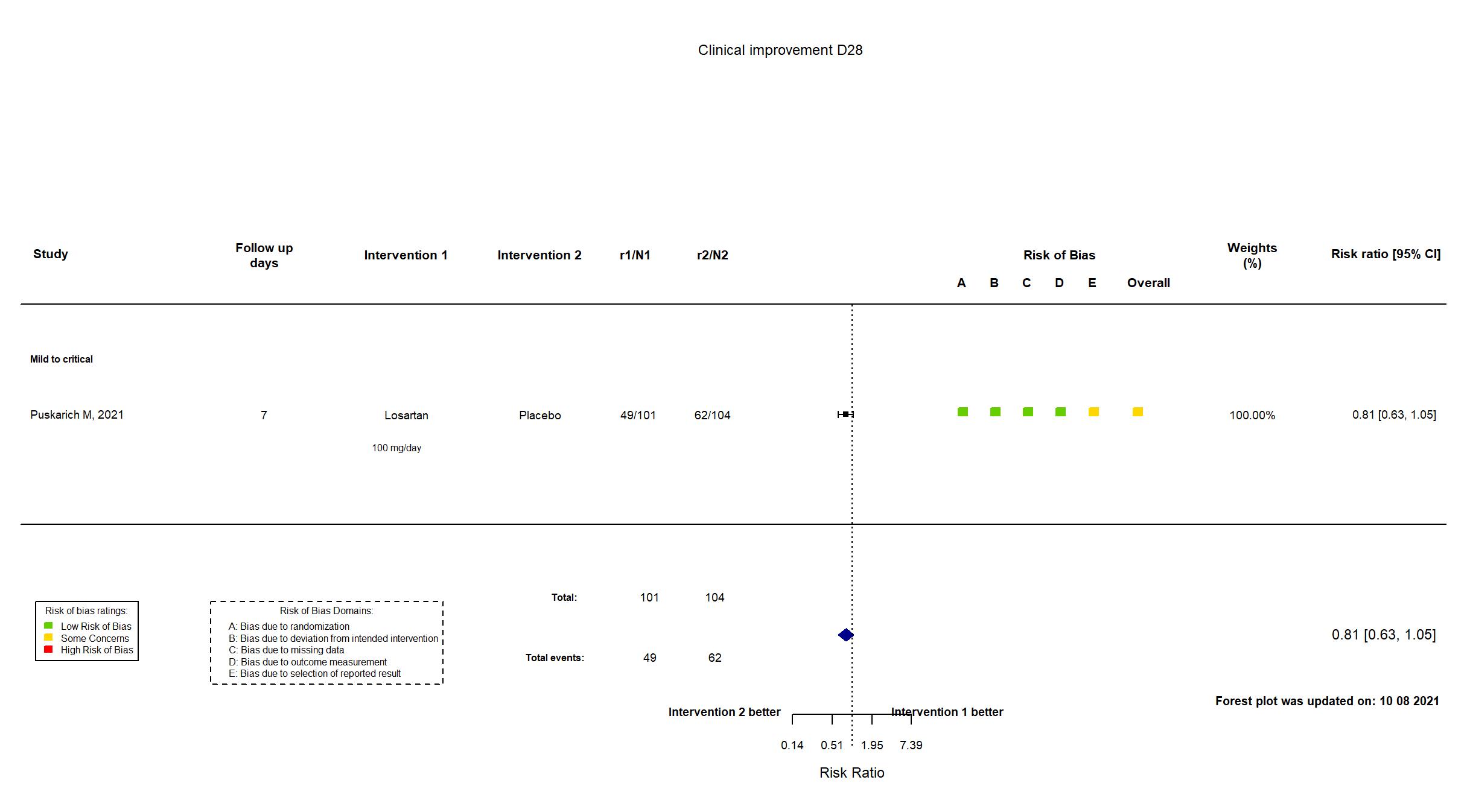
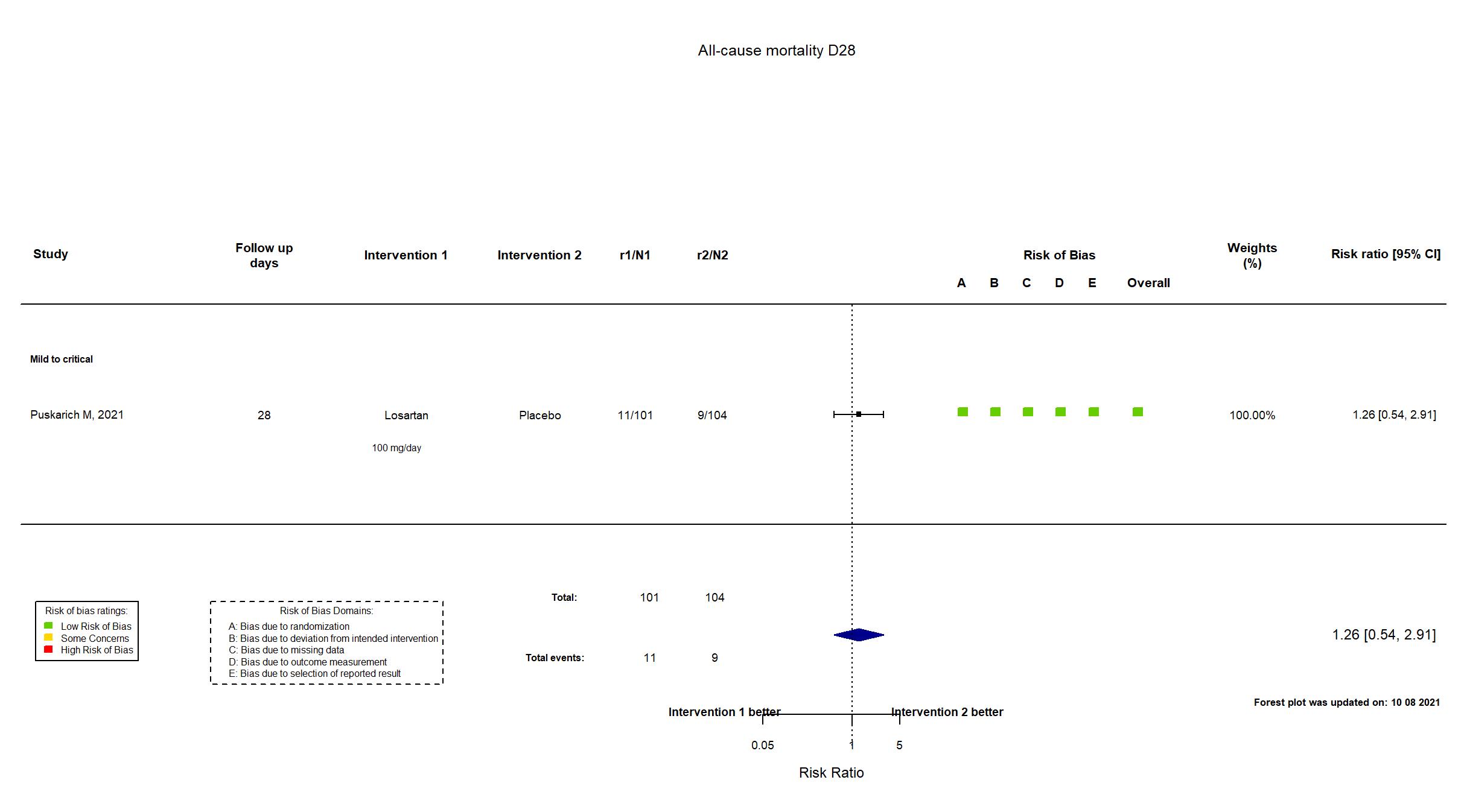
Publication Puskarich M, medRxiv (2021) (preprint)
Dates: 2020-04-01 to 2021-02-28
Funding: Public/non profit (Bill and Melinda Gates Foundation, National Institutes of Health)
Conflict of interest: No
| Methods | |
| RCT Blinding: quadruple blinding | |
| Location :
Multicenter / USA Follow-up duration (days): 90 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Losartan 50 mg orally twice a day if eGFR >60 mL/min/1.73 m2 for 10 days or until discharge; once a day if eGFR 30–60; discontinued if decreased eGFR <30, or if SAE suspected. |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Placebo=104 Losartan =101 | |
| Characteristics of participants N= 205 Mean age : NR 123 males Severity : Mild: n=* / Moderate: n=* / Severe: n=* Critical: n=* | |
| Primary outcome | |
| In the register Difference in Estimated (PEEP adjusted) P/F Ratio at 7 days | |
| In the report PaO2/FiO2 ratio on day 7 | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the pre-print article, the trial registry was used in data extraction and assessment of risk of bias. Neither protocol nor statistical analysis plan was available. The primary outcome in the article reflected that in the registry. Several secondary outcomes in the registry were not reported. The intervention treatment dosage is different in the registry from what was reported in the paper. The trial achieved its target sample size. |