Colchicine vs Standard care/Placebo (RCT)
Mild outpatients
FOREST PLOTS -2022-04-29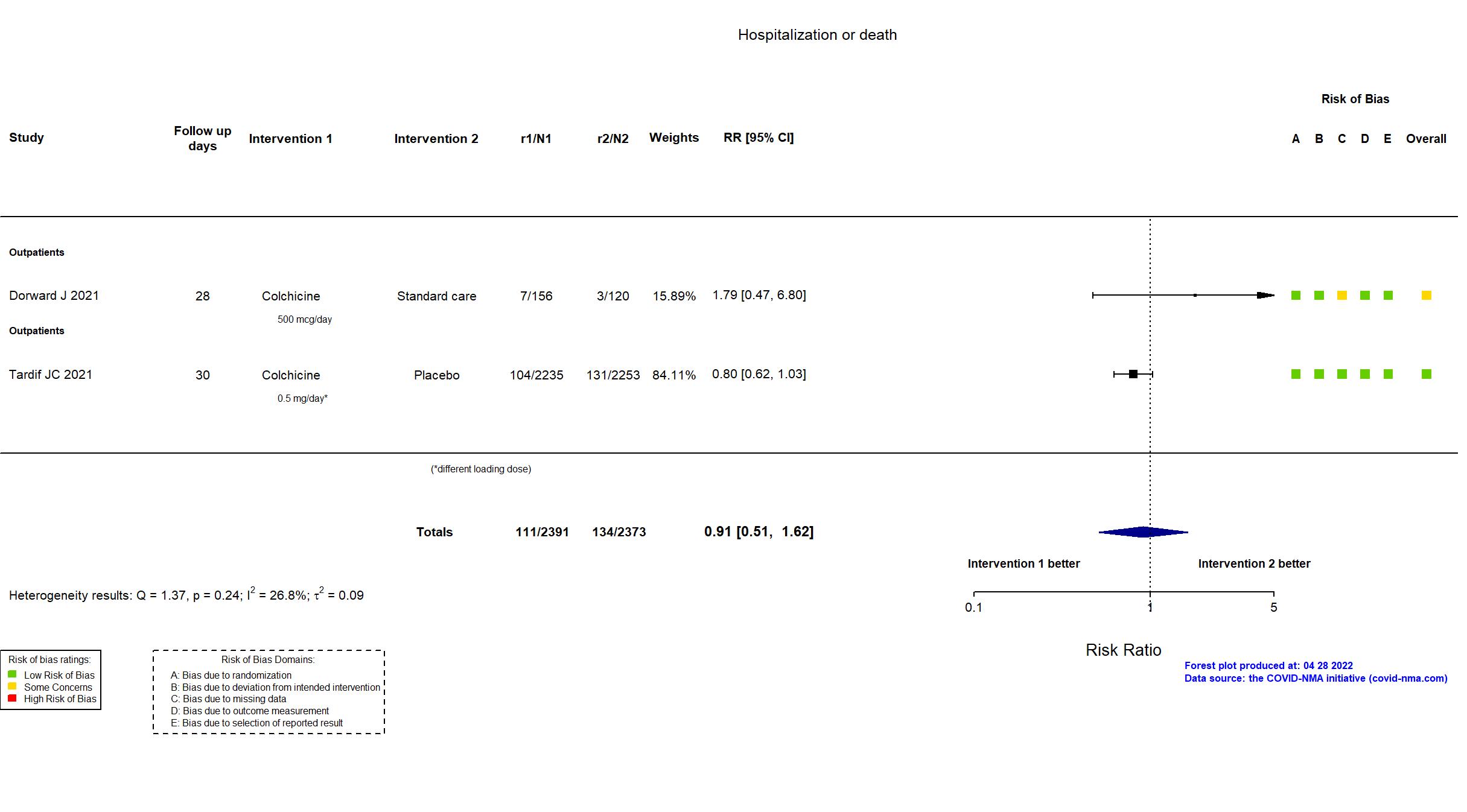
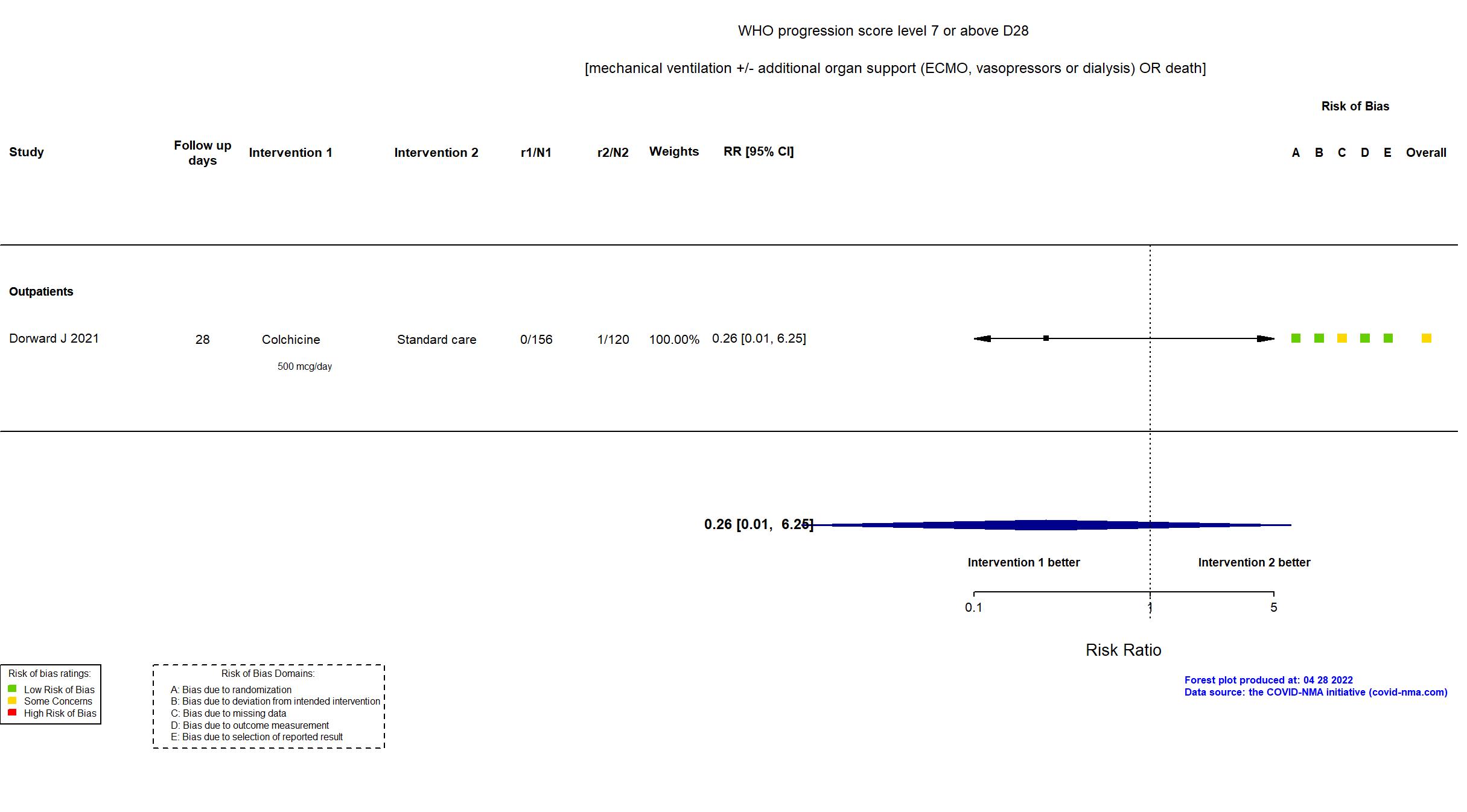
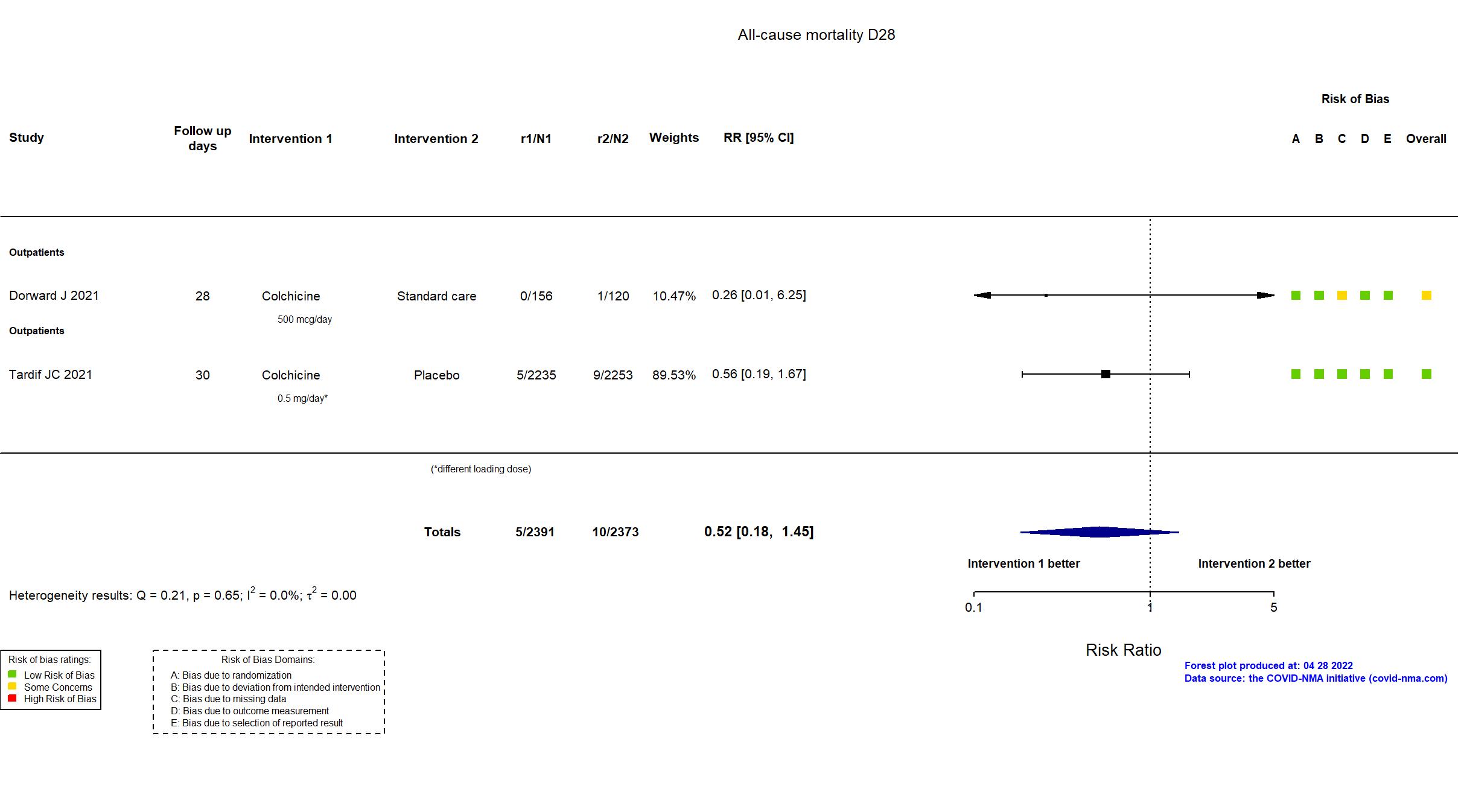

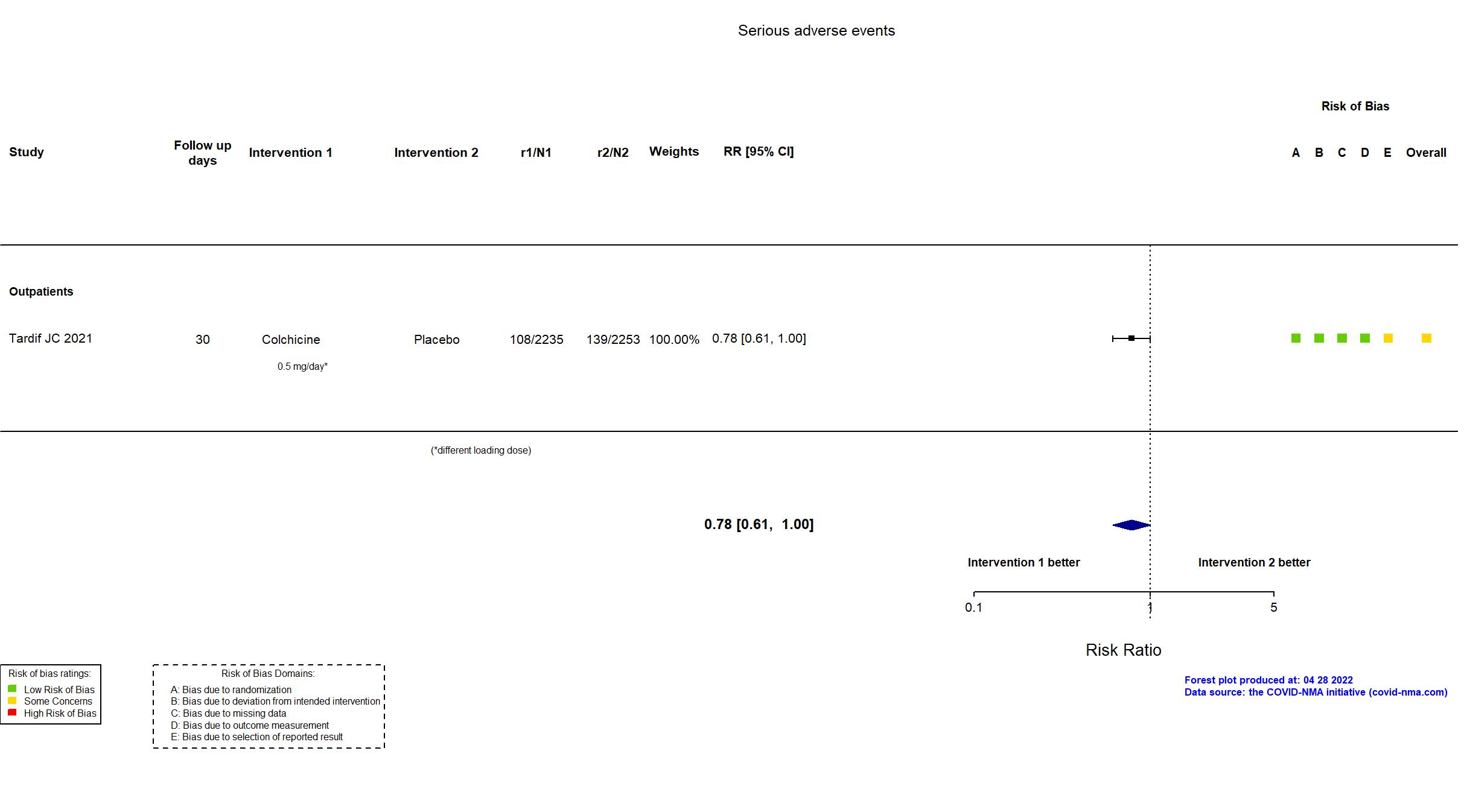





Trial ISRCTN86534580
Publication PRINCIPLE - Dorward J, medRxiv (2021) (preprint)
Dates: 2021-03-04 to 2021-05-26
Funding: Public/non profit (UK Research and Innovation and the Department of Health and Social Care through the National Institute for Health Research)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / UK Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Colchicine 500 mcg orally once a day for 14 days |
|
| Control
Standard care | |
| Participants | |
| Randomized NR Analyzed 276 participants Colchicine=156 Standard care=120 | |
| Characteristics of participants N= 276 Mean age : NR 145 males Severity : Mild: n= 314/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register Hospital admission or mortality related to suspected COVID-19. | |
| In the report 1) time to first reported recovery defined as the first instance that a participant reports feeling recovered; and 2) hospitalisation or death related to COVID-19 | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the pre-print article, the trial registry, protocol, statistical analysis plan and supplementary results were used in data extraction and assessment of risk of bias. The primary outcome indicated in registry does not reflect the primary outcome reported in the paper but is reflected in the protocol amendment. The article reports on a colchicine/usual care comparison that is part of an ongoing multi-treatment platform study. Results have been extracted for the population concurrently randomized to colchicine or usual care to mitigate the potential effect of temporal changes in risk. Recruitment to the colchicine arm was terminated after interim analysis revealed futility. |
Trial NCT04322682
Publication COLCORONA - Tardif JC, Lancet Respir Med (2021) (published paper)
Dates: 2020-03-23 to 2020-12-22
Funding: Public/non profit ( The Government of Quebec, the Bill & Melinda Gates Foundation, the National Heart, Lung, and Blood Institute of the US National Institutes of Health, the Montreal Heart Institute Foundation, the NYU Grossman School of Medicine, the Rudin Family Foundation, and philanthropist Sophie Desmarais.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / Brazil, Canada, Greece, South Africa, Spain, USA Follow-up duration (days): 30 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Colchicine 0.5 mg twice daily days 1-3, then once daily the following 27 days. |
|
| Control
Placebo | |
| Participants | |
| Randomized NR Analyzed 4488 participants Colchicine=2235 Placebo=2253 | |
| Characteristics of participants N= 4488 Mean age : NR 2067 males Severity : Mild: n= */ Asymptomatic: n=* | |
| Primary outcome | |
| In the register Number of participants who die or require hospitalization due to COVID-19 infection [ Time Frame: 30 days post randomization ] | |
| In the report Composite of death or hospitalization due to COVID-19 infection in the 30 days following randomization | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the pre-print article, the prospective trial registry and study website were used in data extraction and assessment of risk of bias. There were no substantive differences between the pre-print article and the trial registry. The study was terminated early due to logistical issues related to maintaining the central study call center active 24 hours per day for a prolonged period of time, as well as the need to provide healthcare systems with study results in a timely fashion given the state of the COVID-19 pandemic. At termination, 75% of the planned patients were recruited and had completed the 30-day follow-up. |