Colchicine vs Standard care/Placebo (RCT)
Hospitalized patients
FOREST PLOTS -2022-06-10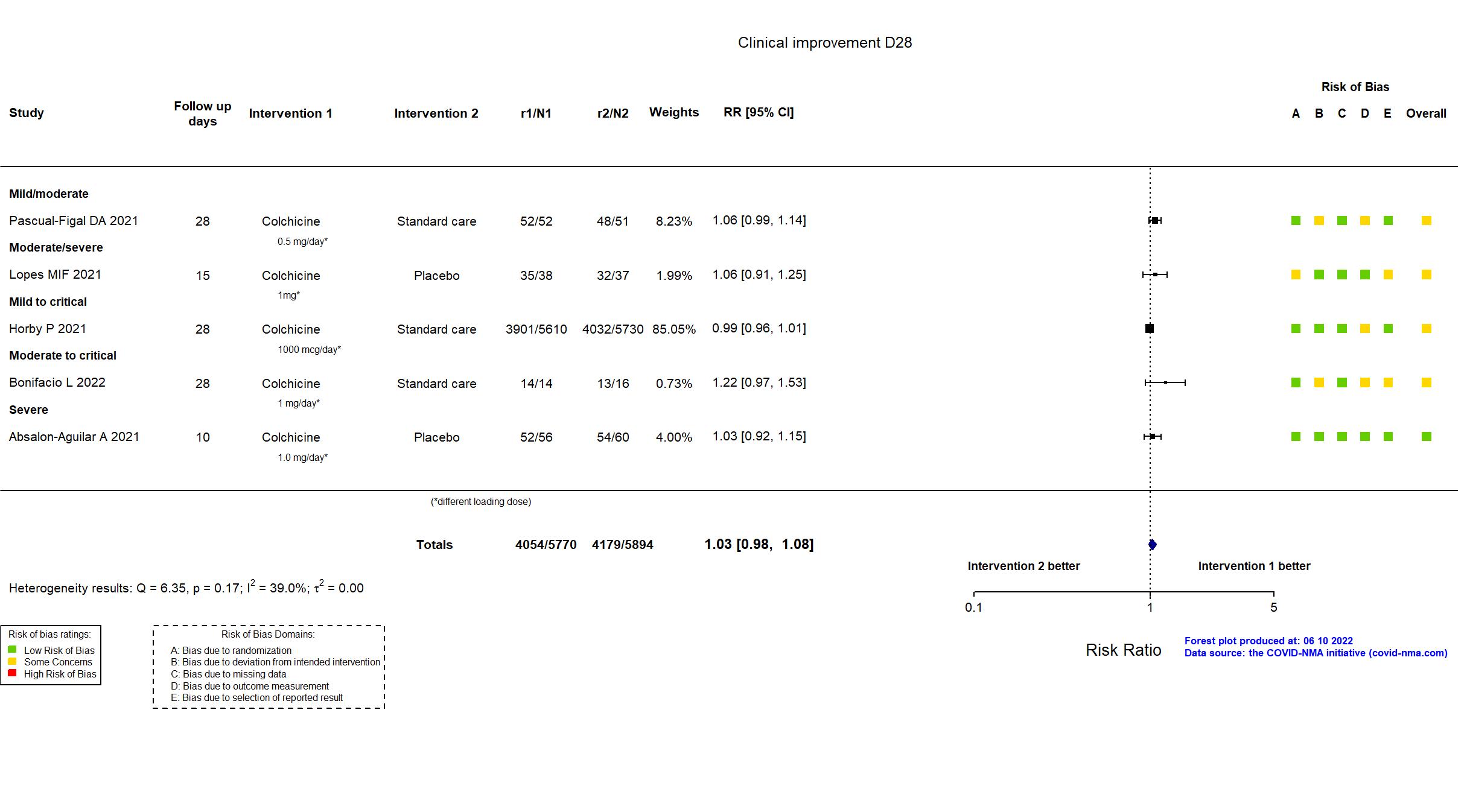
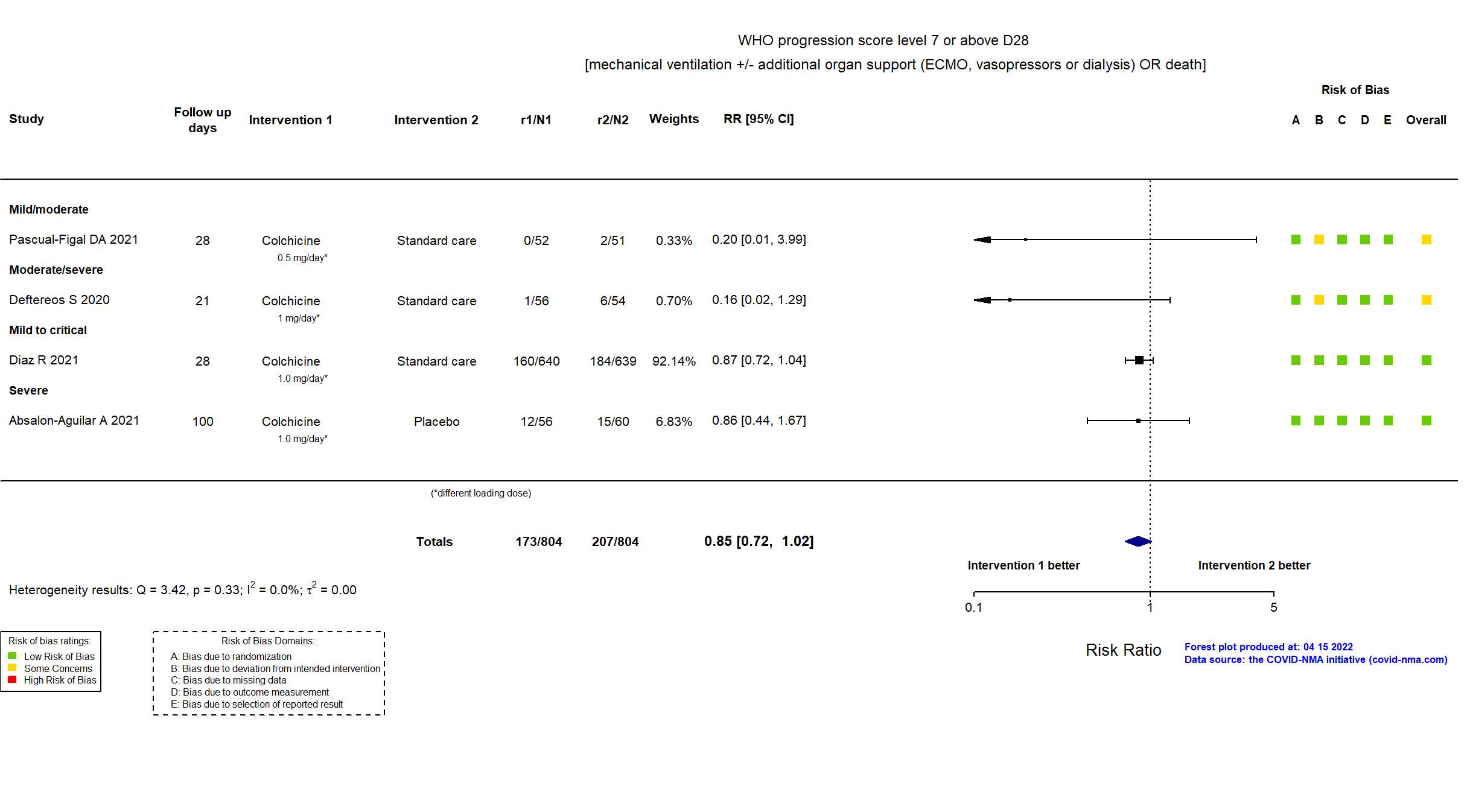
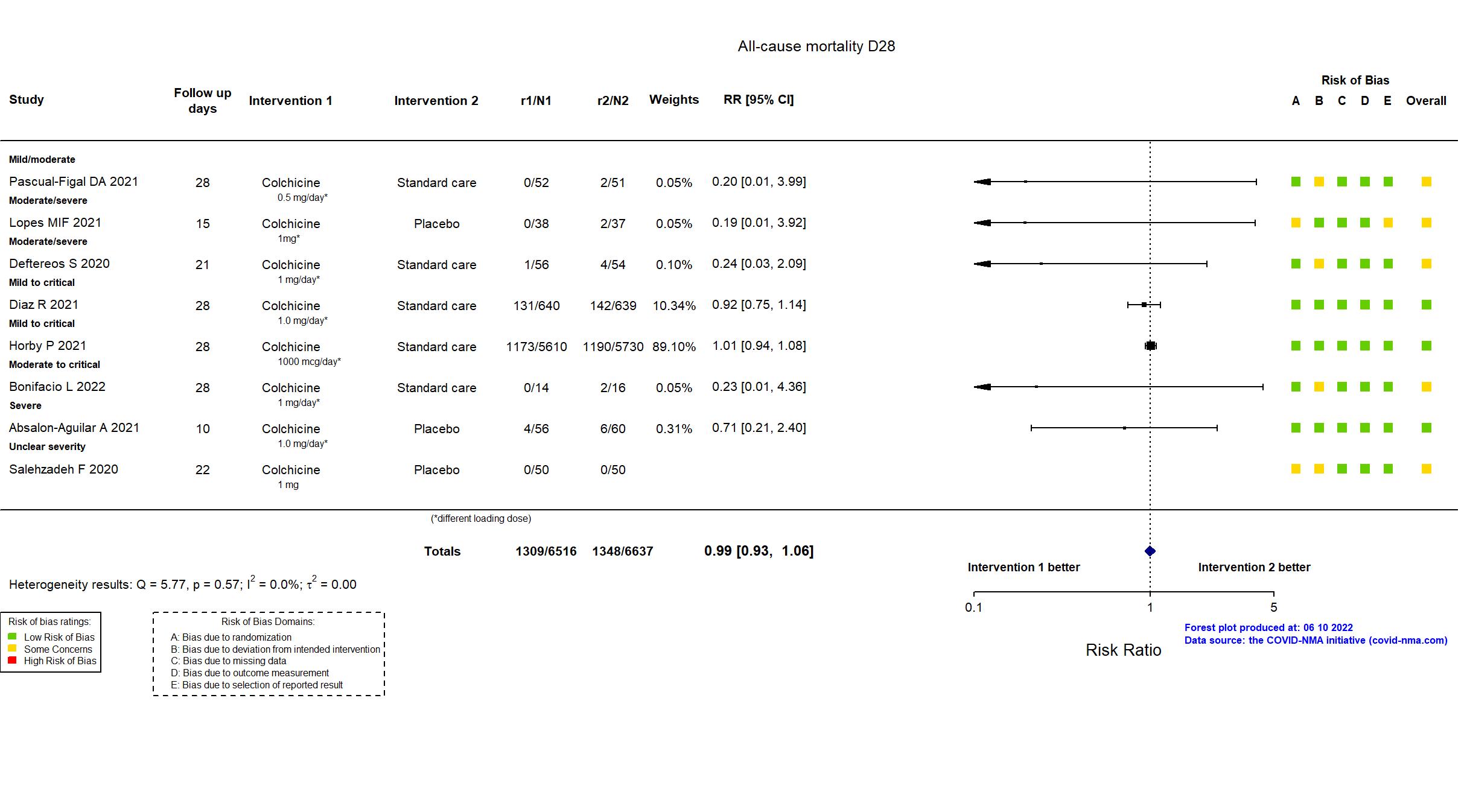
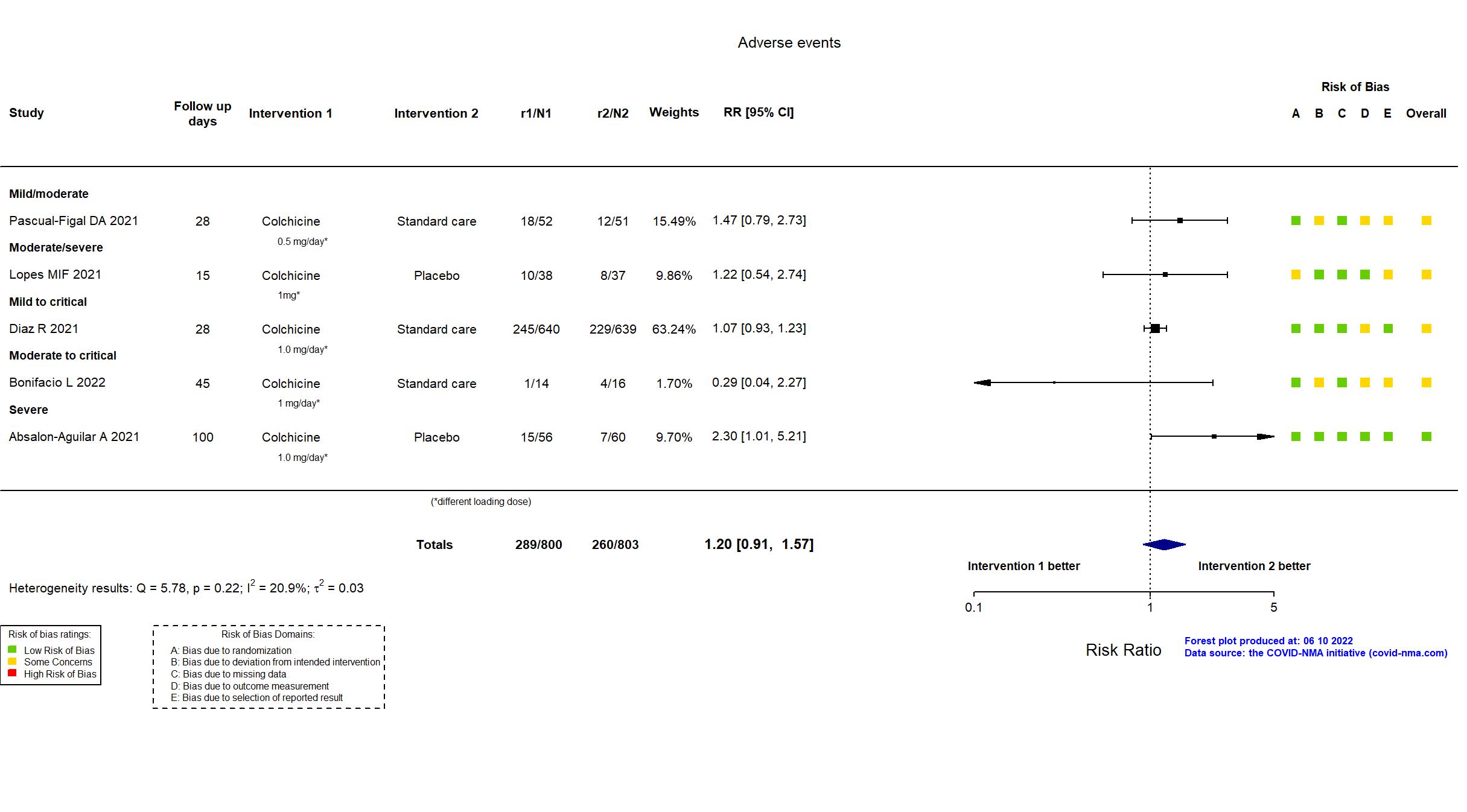








Trial NCT04367168
Publication COLCHIVID - Absalon-Aguilar A, J Gen Intern Med (2021) (published paper)
Dates: 2020-05-01 to 2021-04-30
Funding: Public/non profit (Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán.)
Conflict of interest: No
| Methods | |
| RCT Blinding: triple blinding | |
| Location :
Multicenter / Mexico Follow-up duration (days): 100 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Colchicine Initial dose: 1.5 mg orally once-off - Maintenance dose: 0.5 mg orally twice a day for 10 days. |
|
| Control
Placebo | |
| Participants | |
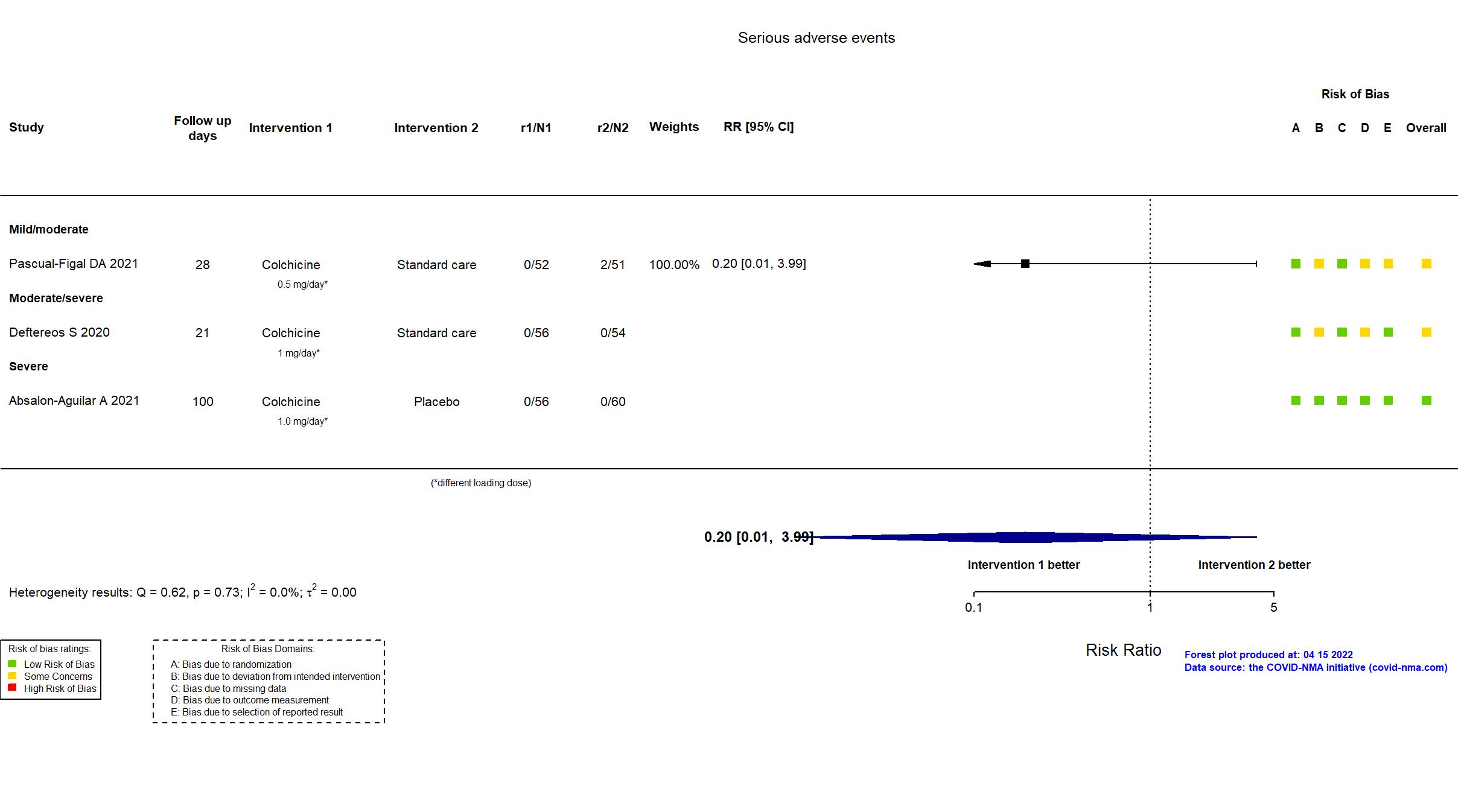
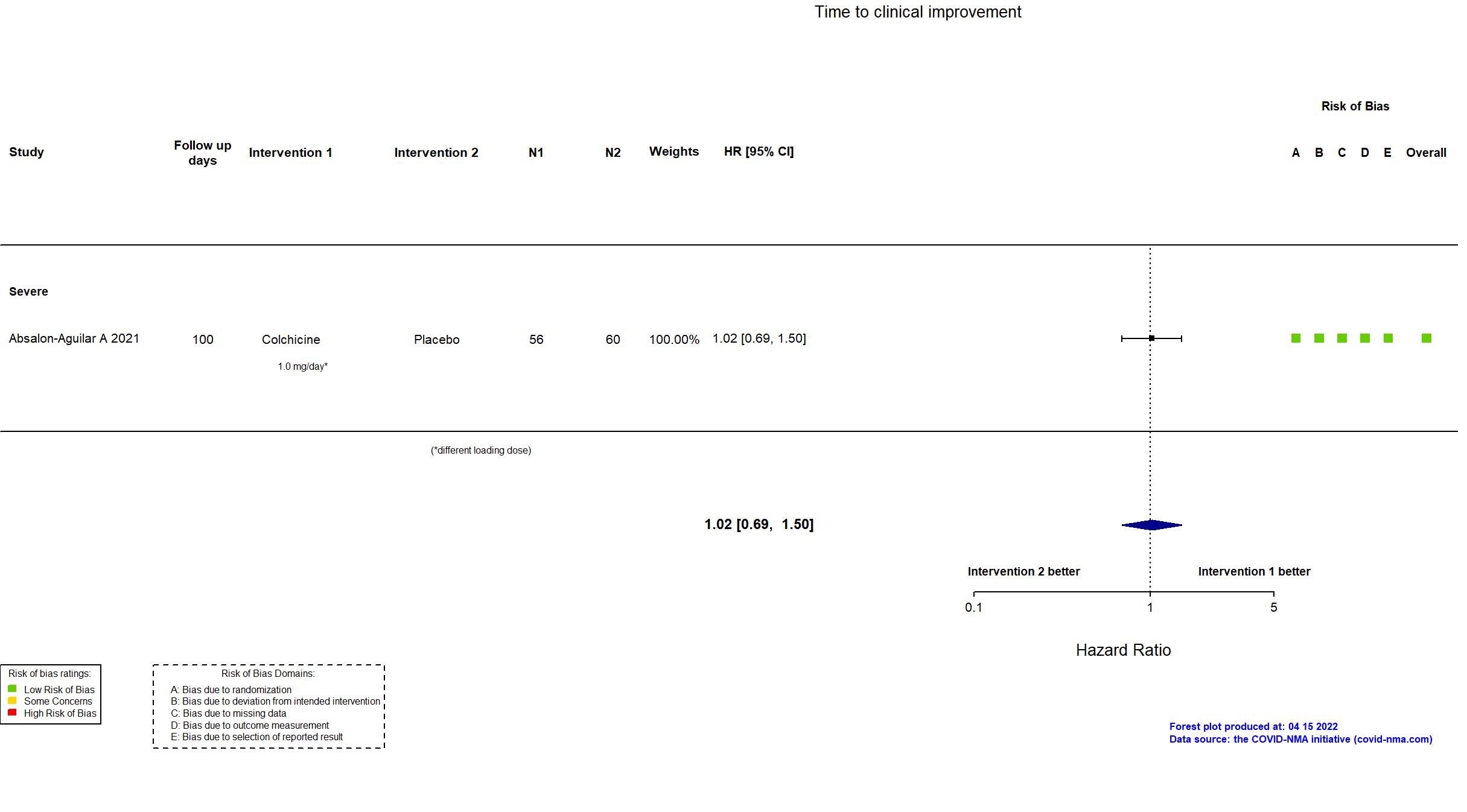
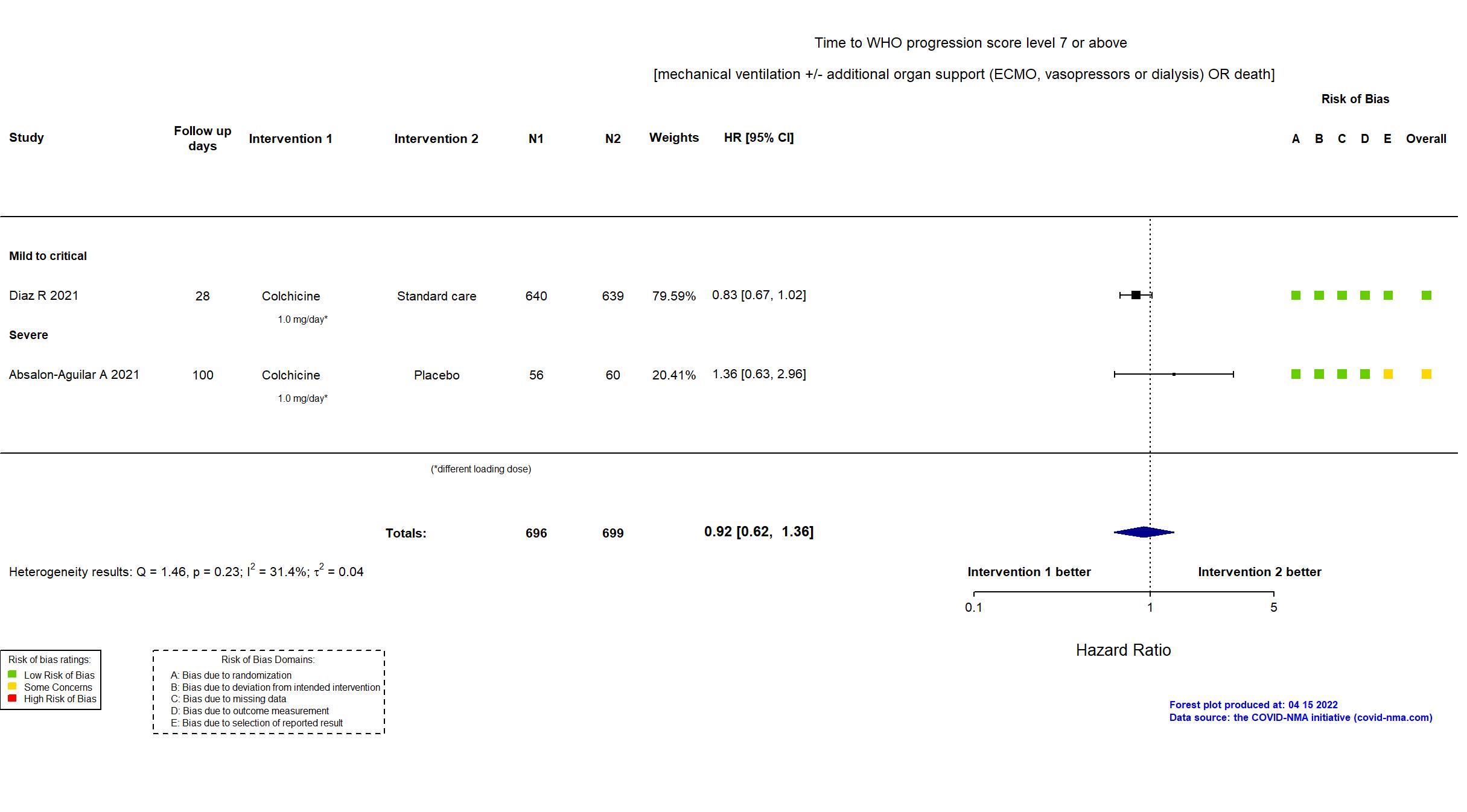
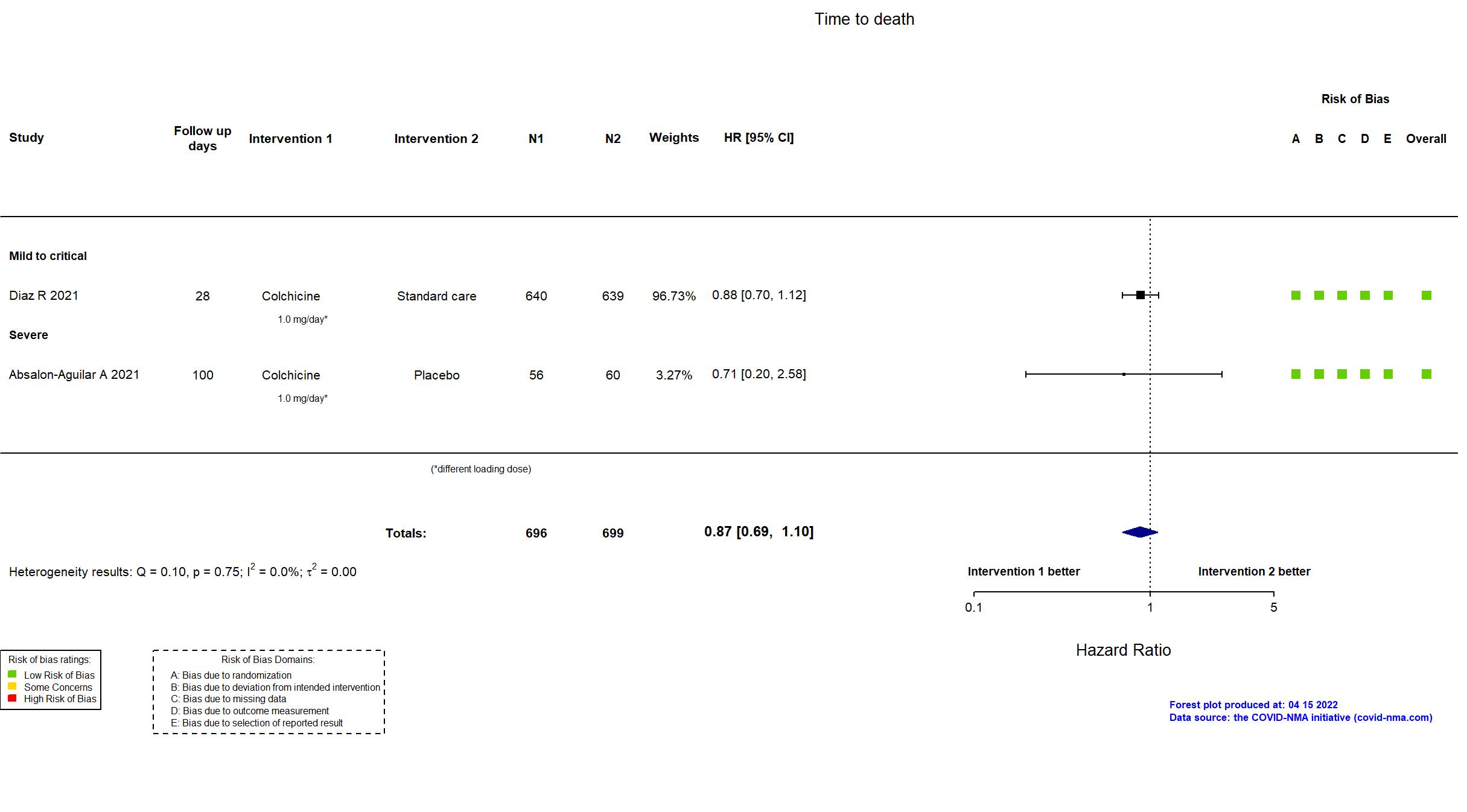
| Randomized participants : Colchicine=56 Placebo=60 | |
| Characteristics of participants N= 116 Mean age : NR 76 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=116 Critical: n=0 | |
| Primary outcome | |
| In the register Number of patients with improvement in body temperature, myalgia, arthralgia, total lymphocyte count, D-dimer, fibrinogen and ferritin levels [ Time Frame: Up to 24 days ] Progression to severe disease [ Time Frame: Up to 10 days ] | |
| In the report Death or progression to critical disease, defined as multiple organ failure, shock, or need for invasive mechanical ventilation. | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published article, the study registry and contact with authors was used in data extraction and risk of bias assessment. The protocol and statistical analysis plan were not available. The registry primary outcome does not reflect the reported primary outcome. Some outcomes in the paper (e.g. adverse events, mortality) were not in the registry but were defined in the protocol. There is no change from the trial registration in the intervention and control treatments, however the inclusion criteria has both mild and severe patients in the registry, while the paper only included severe patients. The study (n=116) did not achieve the target sample size specified in the trial registry (n=174) as the trial was terminated early because the treatment showed not effect on the primary outcome after the second interim analysis.
The study was updated on April 13th, 2022 with data gained from contact with authors. |
Trial *
Publication Alsultan M, Interdiscip Perspect Infect Dis (2021) (published paper)
Dates: 2021-08-01 to 2021-08-30
Funding: Not reported/unclear
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / Syria Follow-up duration (days): * | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Colchicine Initial dose : 1.5 mg orally followed by 0.5 mg 1 hour later on day 1, Maintenance dose: 0.5 mg orally twice daily for 4 days Budesonide 200 mcg inhaled twice daily for 5 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Colchicine=14 Budesonide =14 Standard care=21 | |
| Characteristics of participants N= 49 Mean age : NR 19 males Severity : Mild: n=0 / Moderate: n=* / Severe: n=* Critical: n=0 | |
| Primary outcome | |
| In the register NR | |
| In the report NR | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
* |
| General comment | Only the published article was available for data extraction and assessment of risk of bias. No registry, protocol or statistical analysis plan was available. No outcome was identified as primary in the article. It is not clear whether the study achieved a target sample size. No outcomes with clear time points relevant for COVID-NMA were reported. |
Trial NCT04724629
Publication STRUCK - Bonifacio L, SSRN (2022) (preprint)
Dates: 2021-01-06 to 2021-07-09
Funding: Mixed (Rede Vírus (MCTI - Ministerio Da Ciencia, Tecnologia e Inovacoes) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) (National Council for Scientific and Technological Development). Company Clinigen provided bottles of Interleukin-2 (ProleukinTM) free of charge.)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Brazil Follow-up duration (days): 45 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Ixekizumab 80 mg/ week subcutaneously, once a week for 4 weeks or until discharge. Interleukin-2 1.5 million IU/day subcutaneously for 7 days or until discharge. Colchicine Initial dose: 0.5 mg orally every 8 hours for 3 days - Maintenance dose: 0.5 mg orally twice daily for 4 weeks (+/-7 days) |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Ixekizumab=16 Interleukin-2=14 Colchicine=14 Standard care=16 | |
| Characteristics of participants N= 60 Mean age : NR 37 males Severity : Mild: n=0 / Moderate: n=37 / Severe: n=17 Critical: n=6 | |
| Primary outcome | |
| In the register Ordinal scale of seven World Health Organization (WHO) categories of IL-17 inhibitor versus low dose IL-2 versus indirect IL-6 inhibitor (colchicine) versus standard treatment in the treatment of severe COVID-19 [ Time Frame: On the 21st day of study, since inclusion. ] proportion of patients with clinical improvement, defined by an increase of two points in the ordinal scale of seven WHO categories | |
| In the report Proportion of patients with clinical improvement, defined by an increase of two points on the WHO’s ordinal scale at day 28. | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the preprint and the published conference abstract, the study registry was used in data extraction and risk of bias assessment. Neither the protocol nor statistical analysis plan were available. There is no change from the trial registration in the intervention and control treatments. The timeframe for the registry primary outcome (21 days) does not reflect the reported timeframe for the primary outcome (28 days). Many outcomes from the registry are not reported in the abstract (e.g. need for mechanical ventilation). The preprint reports on an interim analysis and the study achieved the target sample size (n=60) specified in the trial registry.
This study was updated on June 9th 2022 with data from the preprint. |
Trial NCT04326790
Publication Deftereos S, JAMA (2020) (published paper)
Dates: 03apr2020 to 27apr2020
Funding: Private (The study was funded by ELPEN Pharmaceuticals, Acarpia Pharmaceuticals, and Karian Pharmaceuticals.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Greece Follow-up duration (days): 21 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Colchicine Initial dose: 1.5 mg orally followed by 0.5 mg an hour later if no adverse effect - Maintenance dose: 0.5 mg twice a day for a maximum of 21 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Colchicine=56 Standard care=54 | |
| Characteristics of participants N= 110 Mean age : NR 61 males Severity : Mild: n=0 / Moderate: n=102 / Severe: n=3 Critical: n=0 | |
| Primary outcome | |
| In the register Clinical deterioration in the semiquantitative ordinal scale suggested by the WHO R&D committee [ Time Frame: 3 weeks ] Time to clinical deterioration (2 levels in the WHO R&D Blueprint scale) Maximal concentration of cardiac troponin [ Time Fr | |
| In the report The coprimary end points of the biochemical phase were the difference in maximal high-sensitivity cardiac troponin (hs cTn) levels between the 2 groups and the time for C-reactive protein to reach levels greater than 3 times the upper reference limit | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to all available versions of the published article, the study registry, protocol, and statistical analysis plan were used in data extraction and risk of bias assessment. There is no change from the trial registration in the intervention and control treatments. Two types of primary outcomes were presented - a biochemical and a clinical. The biochemical primary outcome in the report partially reflects the primary outcome indicated in the registry. Stated in the registry, it was "Maximal concentration of cardiac troponin [ Time Frame: 10 days ], Maximal concentration of high-sensitivity cardiac troponin" while in the report it was "the difference in maximal high-sensitivity cardiac troponin (hs cTn) levels between the 2 groups and the time for C-reactive protein to reach levels greater than 3 times the upper reference limit." The study did not reach its recruitment target of 180 patients as specified in the registry and protocol "because of slow enrollment as a result of the rapid flattening of the curve of COVID-19 cases in Greece." |
Trial NCT04328480
Publication ECLA PHRI COLCOVID - Diaz R, JAMA Netw Open (2021) (published paper)
Dates: 2020-04-17 to 2021-03-28
Funding: Mixed (The Population Health Research Institute contributed fees to the investigators. Fundacion ECLA funded all other aspects of the trial. Colchicine was donated by Spedrog Caillon to some centers lacking it.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Argentina Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Colchicine Initial dose: 1.5 mg orally followed by 0.5 mg orally within 2 hours - Maintenance dose: 0.5 mg orally twice a day for 14 days or until discharge. |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Standard care=639 Colchicine=640 | |
| Characteristics of participants N= 1279 Mean age : NR 830 males Severity : Mild: n=196 / Moderate: n=995 / Severe: n=23 Critical: n=65 | |
| Primary outcome | |
| In the register All-cause mortality [ Time Frame: During hospitalization or until death, whichever comes first, assessed up to 30 days ] | |
| In the report 1) Composite of a new requirement for mechanical ventilation or death evaluated at 28 days after randomization; 2) Death assessed at 28 days after randomization. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the trial registry, protocol, statistical analysis plan and supplementary appendices were used in data extraction and assessment of risk of bias. The primary outcome in the article reflected that in the registry, but differed from that in the original protocol. Due to low recruitment and low likelihood of reaching the original estimated sample size of 2500 patients, with the aim of reducing the trial sample size and prior to knowing the results, the executive committee decided to change the single primary outcome to two co-primary outcomes: the original primary outcome (all-cause mortality); a composite secondary outcome (new need for mechanical ventilation or death). The trial (n = 1279) achieved its amended target sample size (n = 1200). |
Trial NCT04381936 ; ISRCTN50189673
Publication RECOVERY (COL) - Horby P, Lancet Respir Med (2021) (published paper)
Dates: 2020-11-27 to 2021-03-04
Funding: Mixed (UK Research and Innovation (Medical Research Council); National Institute of Health Research; Wellcome Trust;. Bill and Melinda Gates Foundation; Foreign, Commonwealth and Development Office; Health Data Research UK; Combiphar (drug donation))
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / UK, Indonesia, Nepal Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Colchicine Initial dose: 1 mg after randomization and 500 mcg 12 hours later - Maintenance dose: 500 mcg twice daily orally or by nasogastric tube for 10 days or until discharge. |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Standard care=5730 Colchicine=5610 | |
| Characteristics of participants N= 11340 Mean age : NR 7909 males Severity : Mild: n=* / Moderate: n=* / Severe: n=3034 Critical: n=529 | |
| Primary outcome | |
| In the register All-cause mortality [ Time Frame: Within 28 days after randomisation ] | |
| In the report 28-day mortality | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the pre-print article, the trial registries, protocol and statistical analysis plan were used in data extraction and assessment of risk of bias. There were no substantive differences between the pre-print article and the registry, protocol or statistical analysis plan on procedures, interventions or outcomes. Whereas in the trial registry (clinicaltrials.gov) colchicine was only allowed in women ≥55 years old, in the report only pregnant women were disallowed from the treatment. Recruitment was stopped on the advice of the independent data monitoring committee following a routine interim analysis that revealed there was no convincing evidence that further recruitment to the colchicine comparison would provide conclusive proof of worthwhile mortality benefit either overall or in any pre-specified subgroup. On October 20th, he extraction and risk of bias were updated with the information from the published article. |
Trial RBR-8jyhxh
Publication Lopes MIF, RMD Open (2021) (preprint)
Dates: 2020-04-11 to 2020-08-30
Funding: Public/non profit (FAPESP, CNPq and CAPES grants.)
Conflict of interest: No
| Methods | |
| RCT Blinding: Double blinded, no restrictions | |
| Location :
Single center / Brazil Follow-up duration (days): 15 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Colchicine 0.5 mg orally, twice daily for 5 days, then 0.5 mg twice daily for 5 days; if body weight ≥80 kg, the first dose was 1.0 mg |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Placebo=37 Colchicine=38 | |
| Characteristics of participants N= 75 Mean age : NR 33 males Severity : Mild: n=0 / Moderate: n=* / Severe: n=* Critical: n=* | |
| Primary outcome | |
| In the register To evaluate the duration of oxygen therapy for both groups, measured in number of days of need of supplemental oxygen by catheter or masks. To evaluate the hospitalization time for both groups, measured in number of days from the admission to th | |
| In the report Time of need for supplemental oxygen; time of hospitalization; need for admission and length of stay in ICU; and death rate and causes of mortality | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to all available versions of the published/pre-print article, the study registry were used in data extraction and risk of bias assessment. No pre-specified statistical analysis plan nor protocol was available at the time but authors state that they will be made available as supplementary files in the journal. There is no change from the trial registration in the primary and secondary outcomes. The trial registry reported the intervention treatments as both chloroquine or hydroxychloroquine plus colchicine and the control treatments as both choloroquine or hydroxychloroquine plus placebo. The pre-print reported the intervention arm as colchicine and the control as placebo with choloroquine or hydroxychloroquine, among other drugs (azithromycin, unfractioned heparin and (if needed) methylprednisolone) as standard of care for the institution.
The study was updated on March 19th, 2021 with data from the RMD Open publication. Results based on previous author contact were not considered, since recruitment was not done. Therefore, all results presented are based on the final and published report. |
Trial NCT04350320 ; EUDRACT 2020-001511-2
Publication COL-COVID - Pascual-Figal DA, Int J Gen Med (2021) (published paper)
Dates: 2020-04-30 to 2020-12-04
Funding: Public/non profit (1) “Cardiology Research group” at the IMIB-Arrixaca and the University of Murcia, Murcia, Spain; 2) Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain. Centro Nacional de Investigaciones Cardiovasculares (CNIC) is supported by the Spanish Ministry of Economy and Competitiveness (MINECO) and Pro-CNIC Foundation.)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
* / Spain Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Colchicine Initial dose: 1.5 mg (1 mg and 0.5 mg two hours after) - first maintenance dose: 0.5 mg twice a day for 7 days - second maintenance dose: 0.5 mg once a day for 21 days. (Dose reduced by half in patients receiving ritonavir or lopinavir or with at least one of the following: reduced renal clearance (<50 mL/min/1.37m2), weight <70 kg or age >75 years old.) |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Standard care=51 Colchicine=52 | |
| Characteristics of participants N= 103 Mean age : NR 54 males Severity : Mild: n=34 / Moderate: n=69 / Severe: n=0 Critical: n=0 | |
| Primary outcome | |
| In the register 1) Changes in the patients' clinical status through the 7 points ordinal scale WHO R&D Blueprint expert group [ Time Frame: 7,14,28 Days ]; 2) Changes in IL-6 concentrations [ Time Frame: up to day 28. ] | |
| In the report 1) Change in the WHO 7-points ordinal clinical scale during the 28 days of treatment; 2) effect on IL-6 levels, as main surrogated marker of inflammatory response | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the trial registries and supplementary materials were used in data extraction and assessment of risk of bias. Neither protocol not statistical analysis plan was available. The primary outcomes in the article reflected those in the registry. Some secondary outcomes in the registry were not reported in the article. There was no change from the trial registration in the intervention and control treatments. The study (n = 103) achieved its target sample size (n = 102). |
Trial IRCT20190810044500N5
Publication Pourdowlat G, Phytother Res (2022) (published paper)
Dates: 2020-03-26 to 2020-09-30
Funding: Not reported/unclear
Conflict of interest: No
| Methods | |
| RCT Blinding: single blinding | |
| Location :
Multicenter / Iran Follow-up duration (days): 14 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Colchicine Initial dose: 0.5 mg/day orally for up to 3 days -Maintenance dose: 1 mg/day orally for 12 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Colchicine=102 Standard care=100 | |
| Characteristics of participants N= 202 Mean age : NR 93 males Severity : Mild: n=0 / Moderate: n=* / Severe: n=* Critical: n=0 | |
| Primary outcome | |
| In the register Clinical symptoms including fever, cough, shortness of breath. Timepoint: The first, third, seventh, fourteenth and 6-8 days after entering the study. Method of measurement: questionnaire. Laboratory symptoms (ESR, CRP, NLR, LDH, ferritin, D-dimer, CBC diff). Timepoint: Hospitalization time and discharge time. Method of measurement: Blood test. O2sat at the time of hospitalization and discharge. Timepoint: The first, third, seventh, fourteenth and 6-8 days after entering the study. Method of measurement: Pulse Oximeter. Pulmonary infiltration findings on CT scan. Timepoint: Two weeks later and 6-8 weeks later. Method of measurement: CT-scan. | |
| In the report Clinical status distribution on chest CT evaluations | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: N |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the retrospective study registry was used in data extraction and risk of bias assessment. The protocol and statistical analysis plan were not available. As the registry was retrospective we were unable to determine if the intervention, sample size and analysis plan were determined a-priori. The primary outcome for the paper was unclear, while the registry had four primary outcomes. Some outcomes from the registry are not reported in the paper (e.g., Mortality and Need hospitalization in ICU). |
Trial IRCT20200418047126N1
Publication Salehzadeh F, Research Square (2020) (preprint)
Dates: 2020-05-21 to 2020-06-20
Funding: Public/non profit (Ardabil University of Medical Sciences)
Conflict of interest: No
| Methods | |
| RCT Blinding: | |
| Location :
Single center / Iran Follow-up duration (days): 22 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Colchicine 1 mg orally once a day for 6 days. |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Colchicine=50 Placebo=50 | |
| Characteristics of participants N= 100 Mean age : NR 41 males Severity : Mild: n=0 / Moderate: n=* / Severe: n=* Critical: n=* | |
| Primary outcome | |
| In the register Hospitalization outcome, fever cessation, hospitalization time | |
| In the report (1) Length of hospitalization; (2) symptoms and (3) Co-existed disease. | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the preprint article, the trial registration was used for data extraction and risk of bias assessment. There were considerable differences between outcomes reported in the pre-print article and those in the study registry. Symptoms and co-existed disease were outcomes in the report but they were not specified in the registry. There was a difference in treatment duration between study registry and pre-print article. |