Studies description
Trial NCT04329923
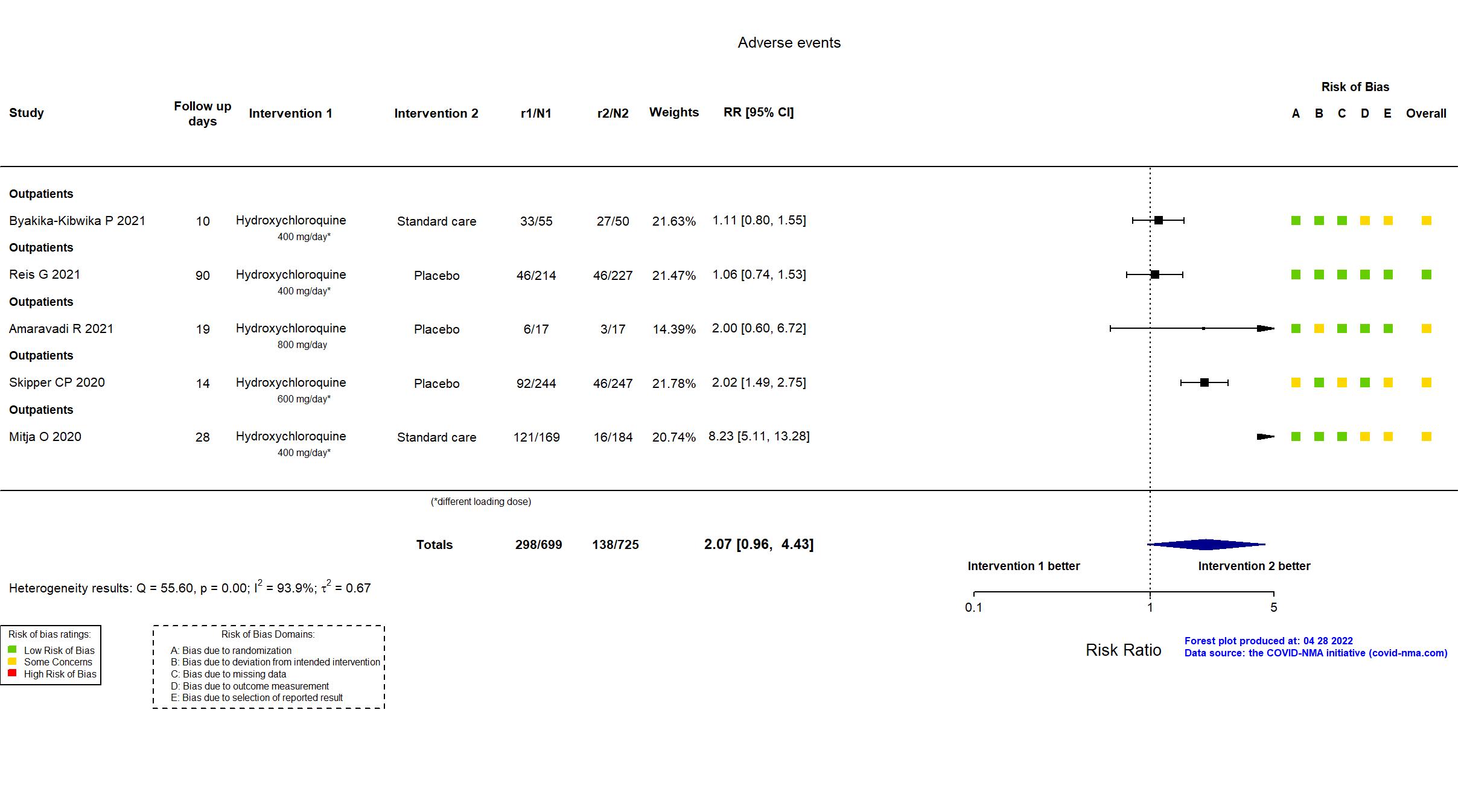
Publication PATCH - Amaravadi R, medRxiv (2021) (preprint)
Dates: 2020-04-15 to 2020-07-14
Funding: Mixed (Leonard & Madlyn Abramson and Mark & Cecilia Vonderheide (philanthropic donations to the University of Pennsylvania) , iRhythm Technologies, Inc. (Cardiac arrhythmia monitoring provided as an in-kind gift))
Conflict of interest: No
Trial NCT04860284
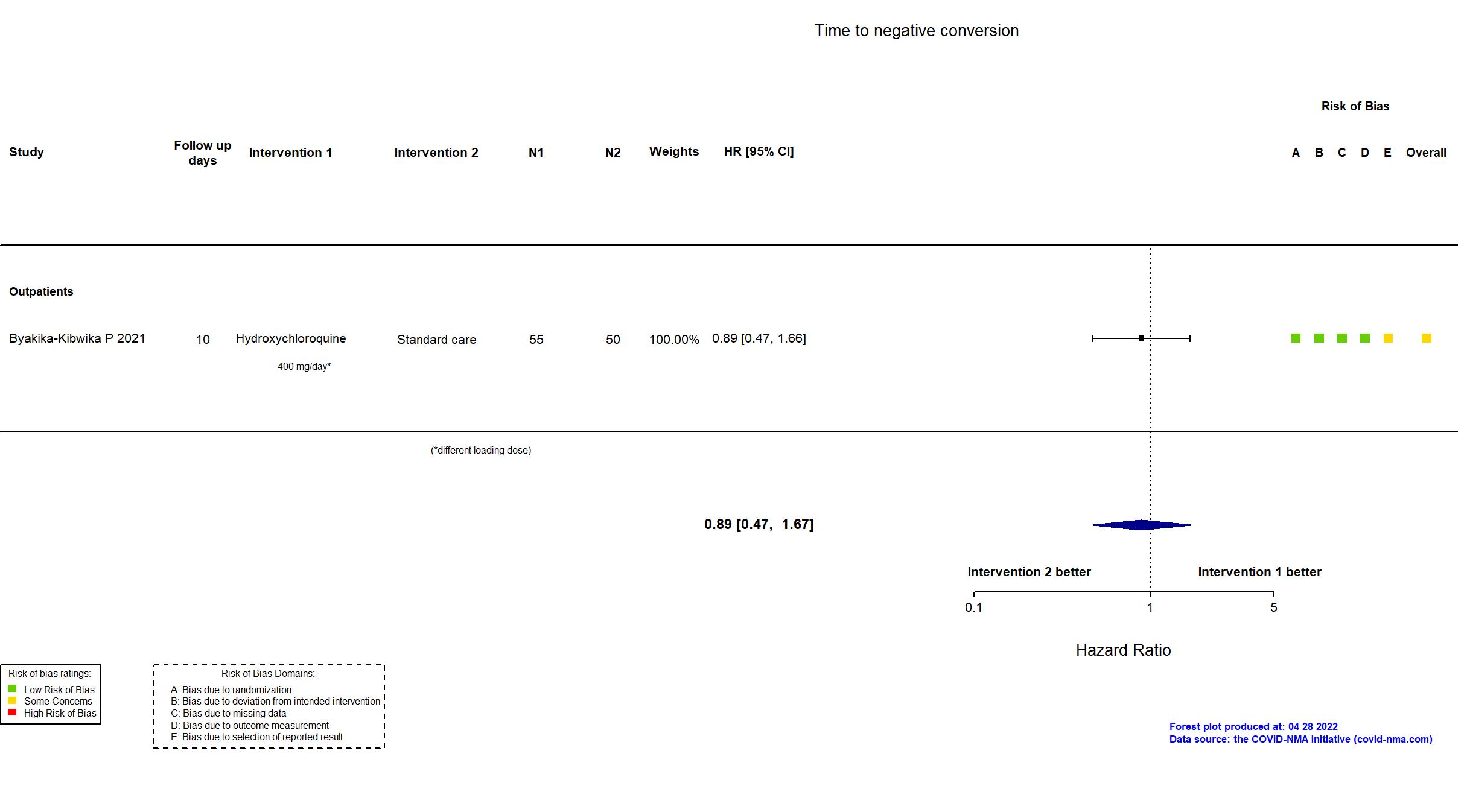
Publication HONEST - Byakika-Kibwika P, BMC Infect Dis (2021) (published paper)
Dates: 2020-09-18 to 2021-02-28
Funding: Public/non profit (Government of Uganda through the Makerere University Research and Innovation Fund)
Conflict of interest: No
Trial NCT04304053
Publication Mitja O, Clin Infect Dis (2020) (published paper)
Dates: 17mar2020 to 26may2020
Funding: Mixed (Crowdfunding campaign JoEmCorono; Laboratorios Rubio, Laboratorios Gebro Pharma, Zurich Seguros, SYNLAB Barcelona, Generalitat de Catalunya)
Conflict of interest: No
Trial NCT04349592
Publication Q-PROTECT - Omrani A, EClinicalMedicine (2020) (published paper)
Dates: 2020-04-13 to 2020-08-01
Funding: Public/non profit (Hamad Medical Corporation (government health service of the State of Qatar))
Conflict of interest: No
Trial NCT04403100
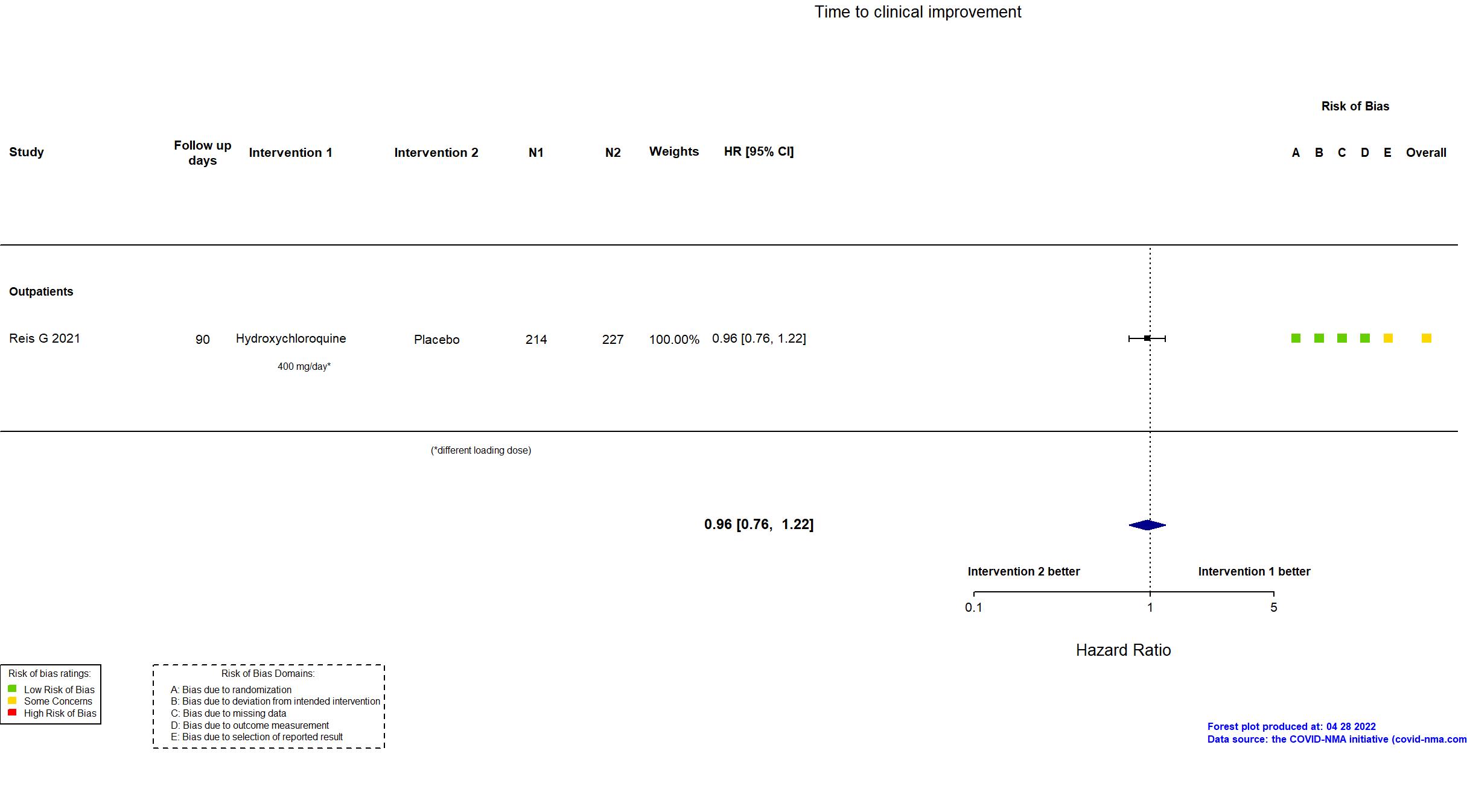
Publication TOGETHER - Reis G, JAMA (2021) (published paper)
Dates: 2020-06-02 to 2020-10-09
Funding: Public/non profit (Bill and Melinda Gates Foundation)
Conflict of interest: No
Trial NCT04329611
Publication Schwartz I, CMAJ Open (2021) (published paper)
Dates: 2020-04-15 to 2020-05-22
Funding: Mixed (Calgary Health Trust, the University of Calgary, Alberta Innovates Health Solutions, Alberta Health Services and the Alberta Government provided funding. Hydroxychloroquine and matching placebo were provided by Apotex.)
Conflict of interest: No
Trial NCT04308668
Publication Skipper CP, Ann Intern Med (2020) (published paper)
Dates: 22-mars-20 to 05-juin-20
Funding: Private (Private donors)
Conflict of interest: Yes