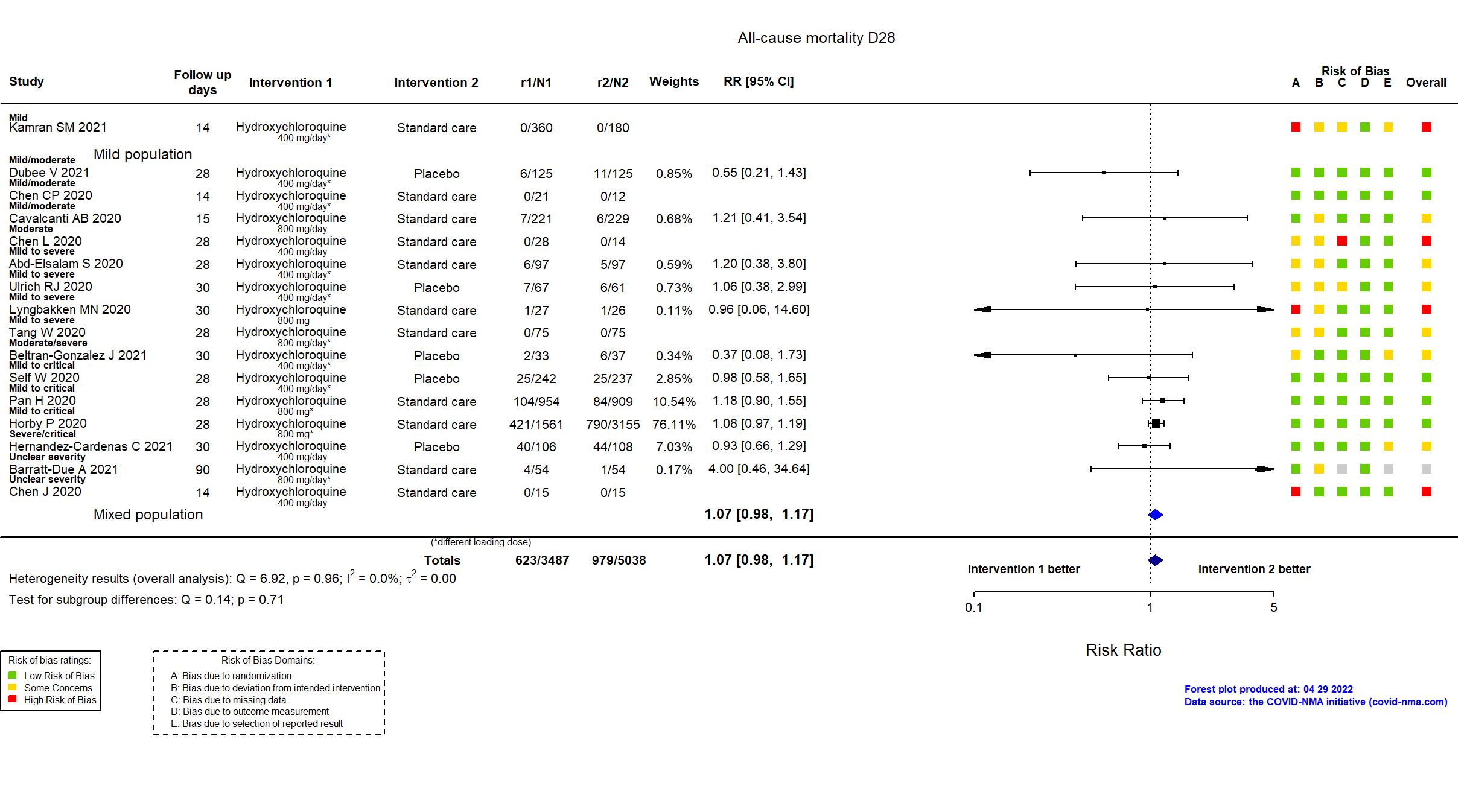
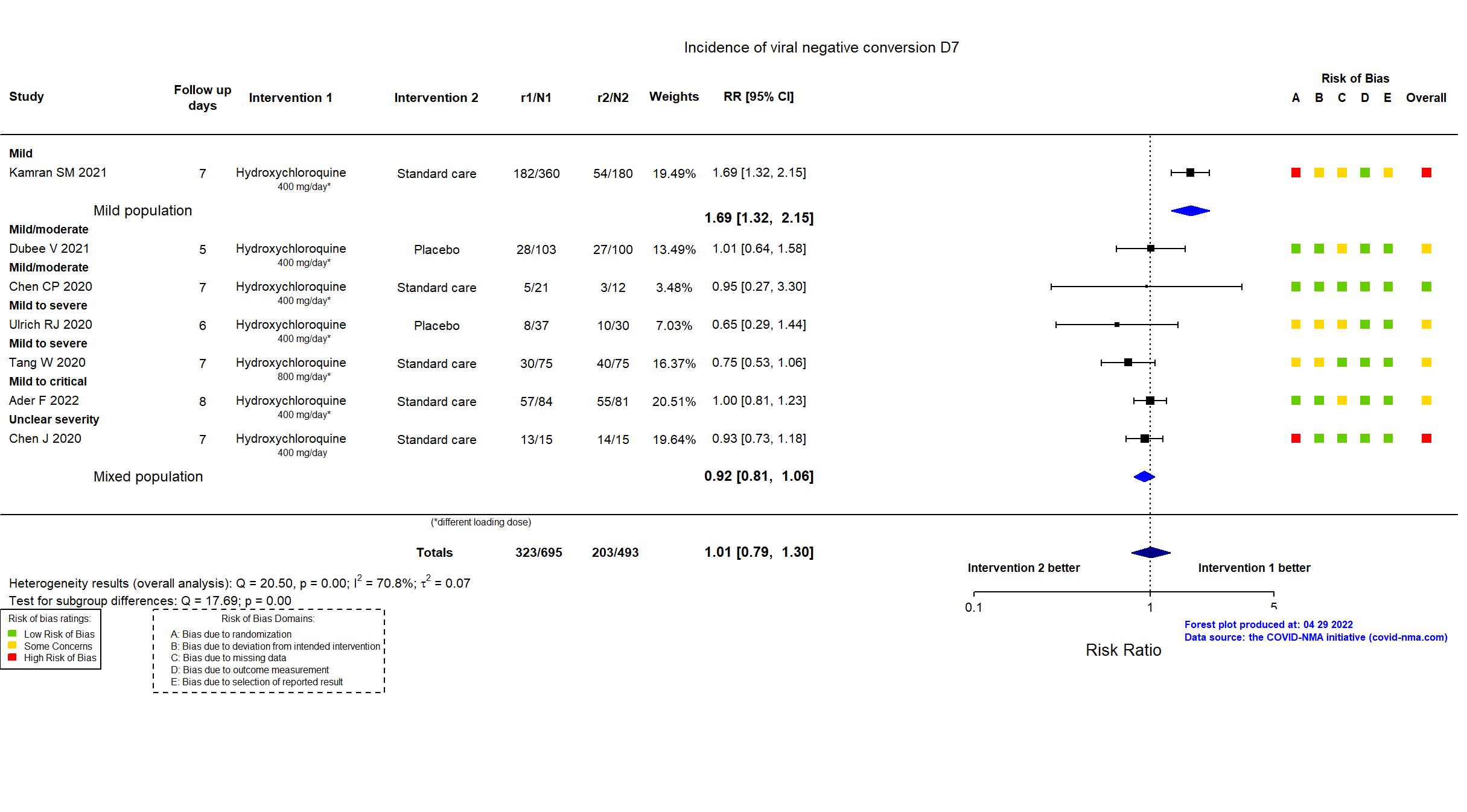
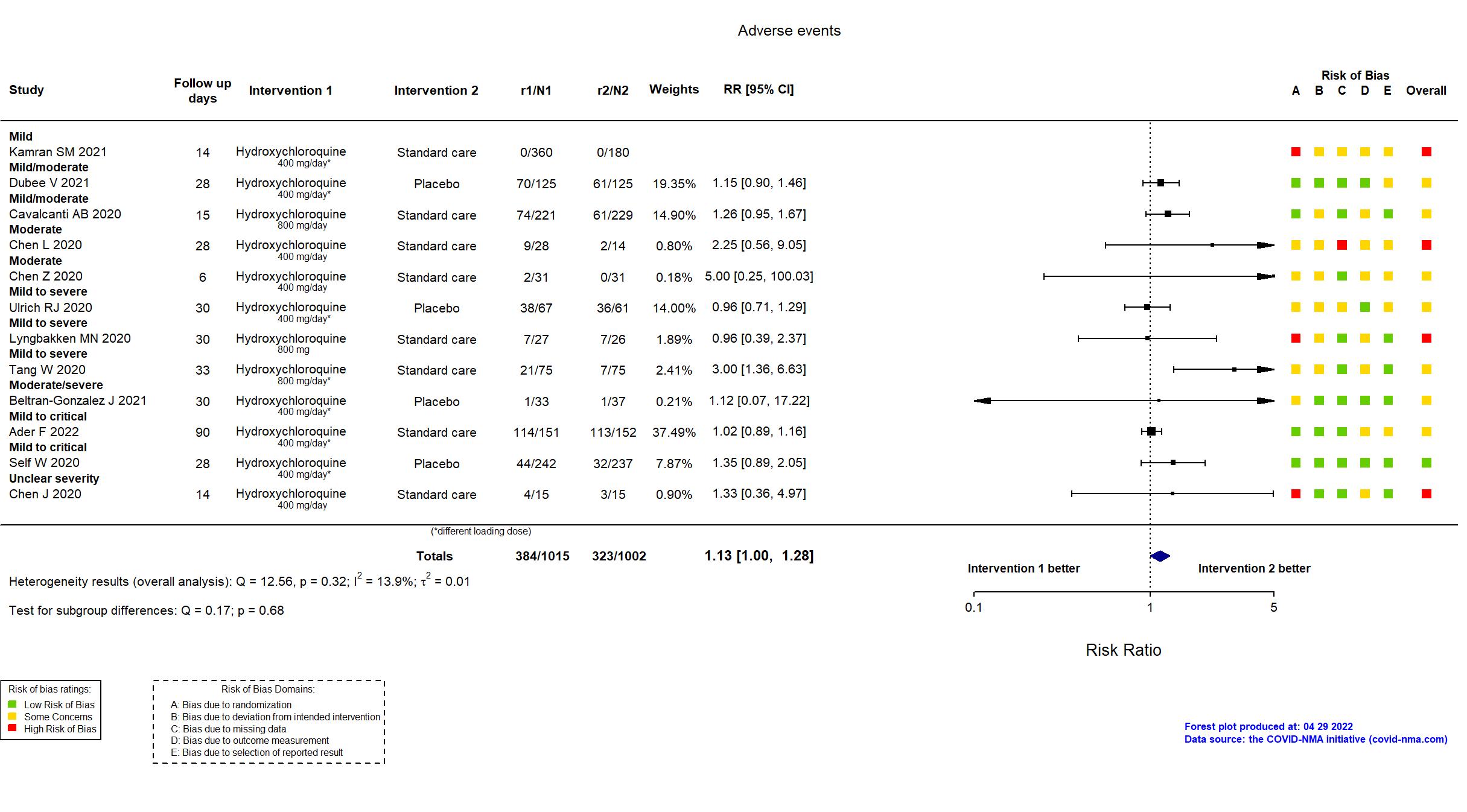
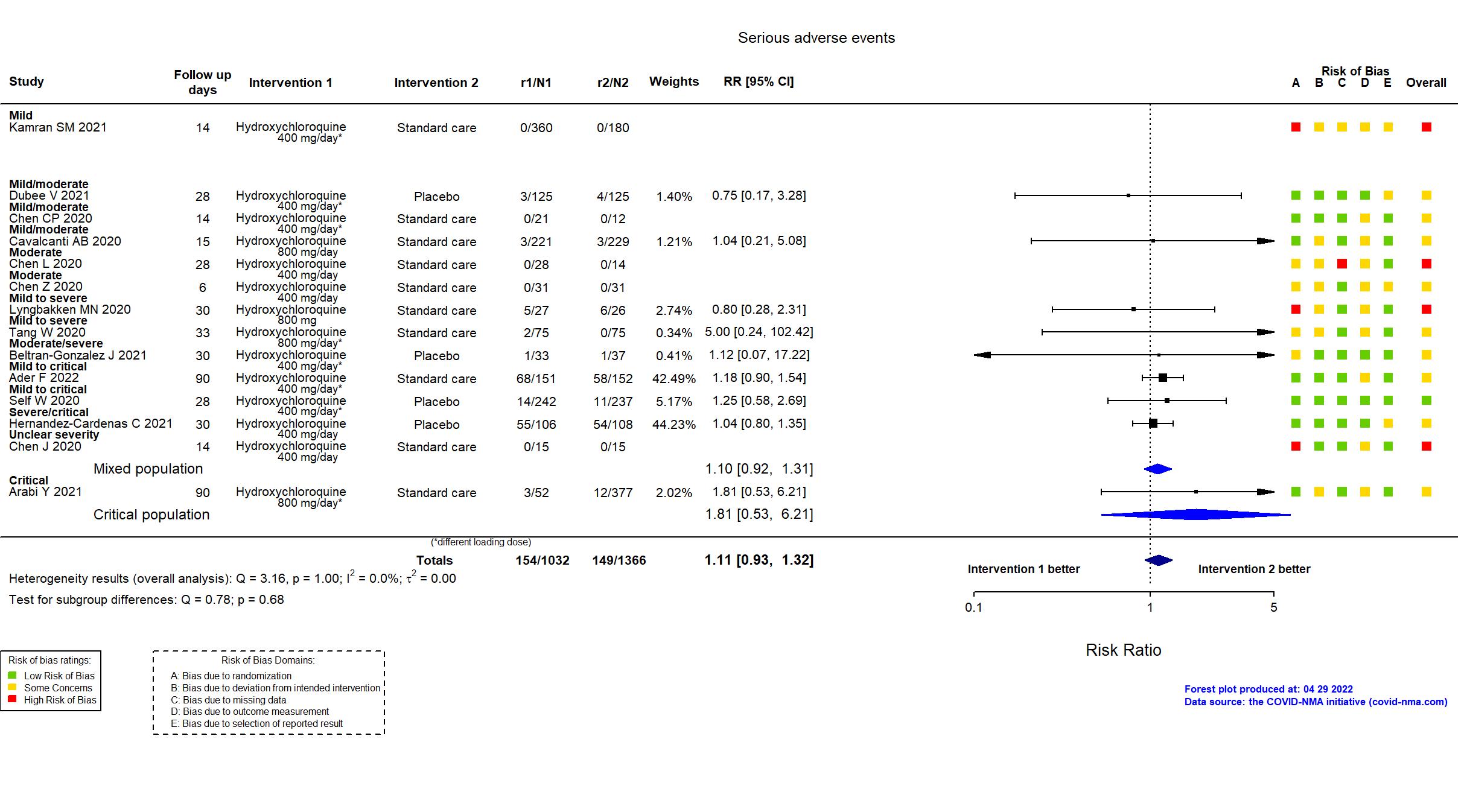
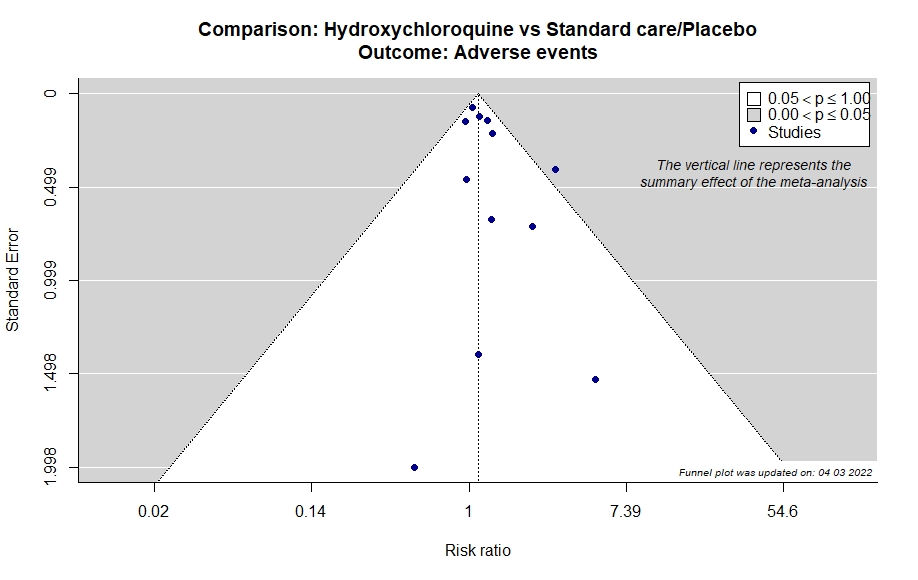
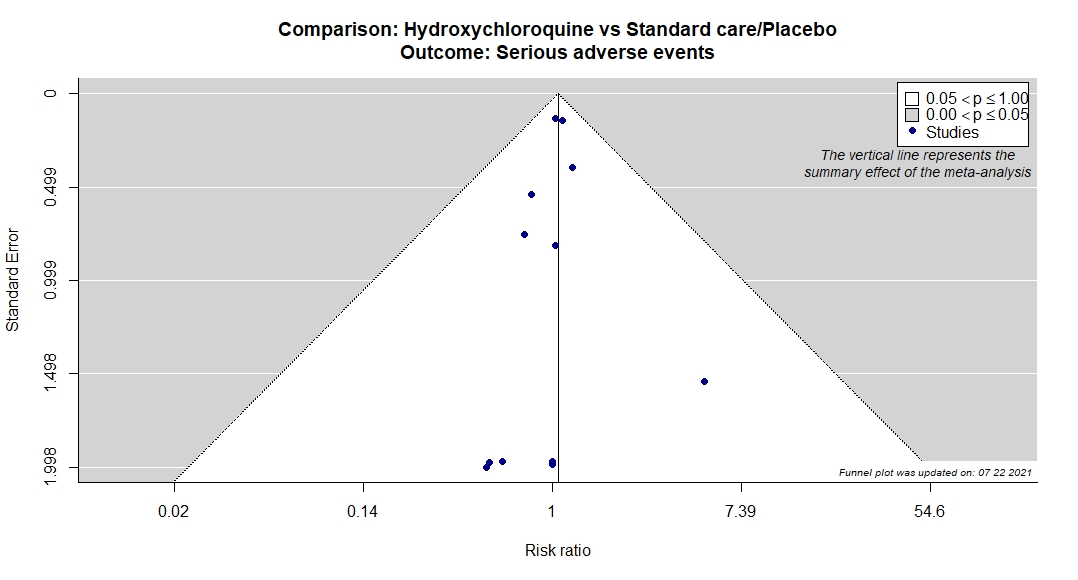
Studies description
Trial NCT04353336
Publication Abd-Elsalam S, Am J Trop Med Hyg (2020) (published paper)
Dates: 2020-03-23 to 2020-06-30
Funding: Public/non profit (Tanta University)
Conflict of interest: *
Trial NCT04315948
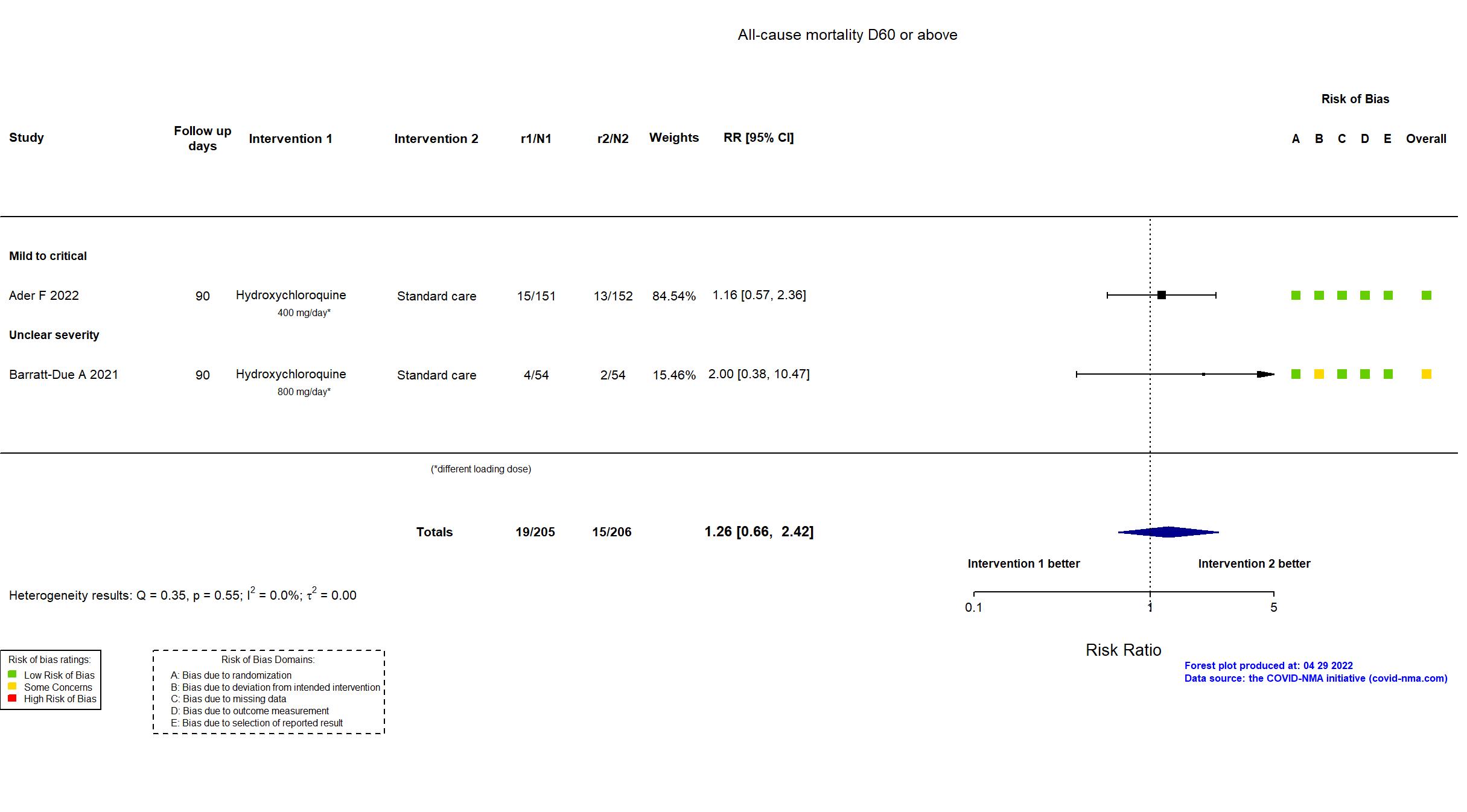
Publication DisCoVeRy - Ader F, medRxiv (2022) (preprint)
Dates: 2020-03-22 to 2020-06-29
Funding: Mixed (Programme Hospitalier de Recherche Clinique, DIM One Health Île-de-France, REACTing, INSERM. GILEAD, SANOFI, MERCK and ABBVIE (drug provision) )
Conflict of interest: Yes
Trial *
Publication Ahmad B, Clin Med Res (2021) (published paper)
Dates: 2020-06-01 to 2020-06-15
Funding: No specific funding (The authors have reported no conflicts of interest or financial support for this work.)
Conflict of interest: No
Trial NCT02735707
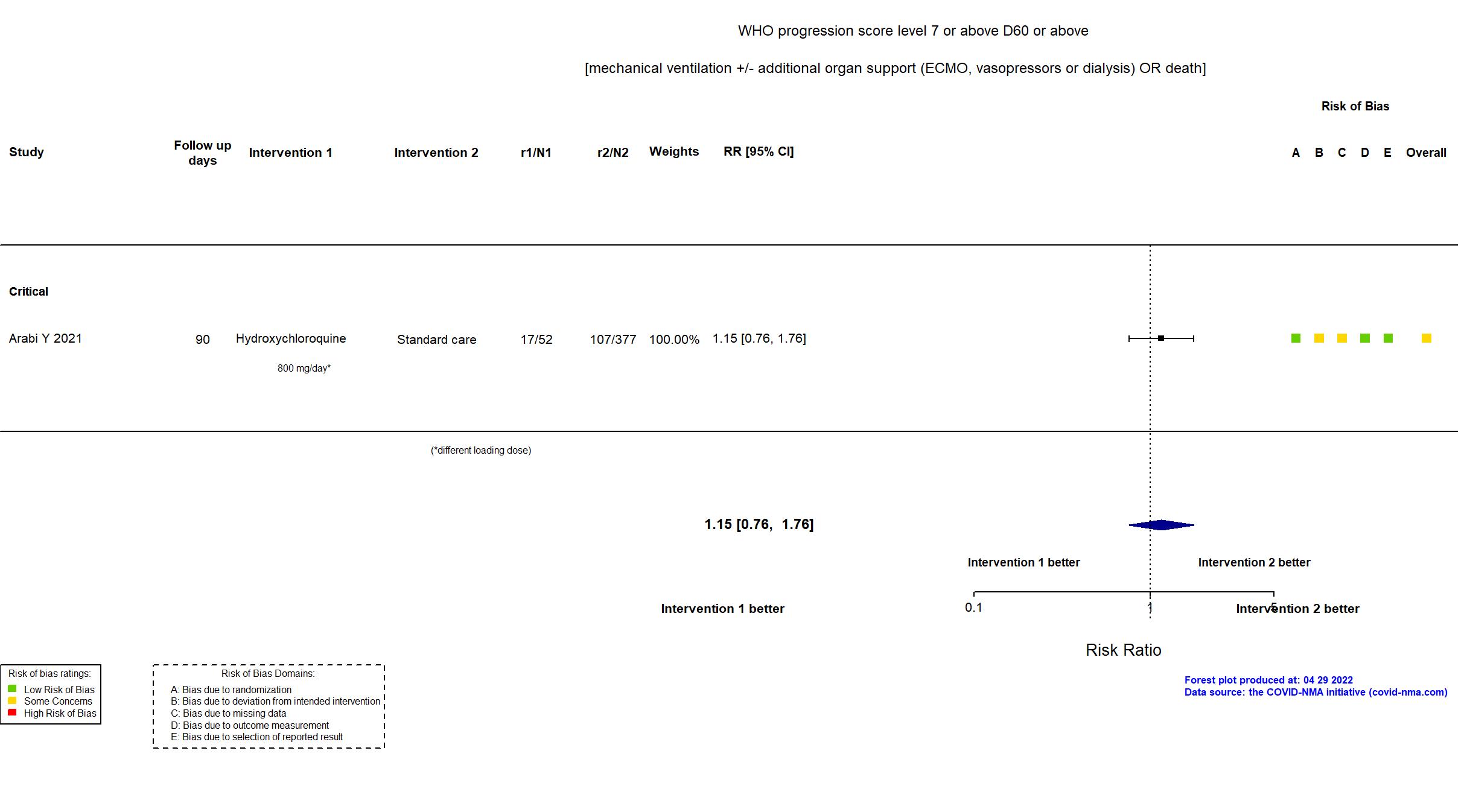
Publication Arabi Y, Intensive Care Med (2021) (published paper)
Dates: 2020-04-08 to 2020-11-19
Funding: Mixed (The European Union through the Platform for European Preparedness Against emerging Epidemics (PRE‑PARE) consortium and Horizon 2020 research and innovation program (the Rapid European Covid-19 Emergency Research response (RECOVER) consortium; the Australian National Health and Medical Research Council; the Health Research Council of New Zealand; Canadian Institutes of Health Research Strategy for
Patient-Oriented Research Innovative Clinical Trials Program Grant; the U.K. NIHR and the NIHR Imperial Biomedical Research Centre; the Health Research Board of Ireland; the UPMC Learning While Doing Program; the Breast Cancer Research Foundation; the French Ministry of
Health; the Minderoo Foundation; Amgen; Eisai; the Global Coalition for Adaptive Research; the Wellcome Trust Innovations Project)
Conflict of interest: No
Trial NCT04321616
Publication NOR-SOLIDARITY - Barratt-Due A, Ann Intern Med (2021) (published paper)
Dates: 2020-03-28 to 2020-06-08
Funding: Mixed (National Clinical Therapy Research in the Specialist Health Services, Norway; Mylan (drug donation)
)
Conflict of interest: No
Trial NCT04391127
Publication Beltran-Gonzalez J, medRxiv (2021) (preprint)
Dates: 2020-05-04 to 2020-08-15
Funding: Public/non profit (Aguascalienes State Health Institute)
Conflict of interest: No
Trial NCT04322123
Publication Cavalcanti AB, N Engl J Med (2020) (published paper)
Dates: 3/29/2020 to 5/17/2020
Funding: Mixed (Hospitals and research institutes participating in Coalition Covid-19 Brazil; EMS Pharma )
Conflict of interest: Yes
Trial NCT04384380
Publication Chen CP, Plos one (2020) (published paper)
Dates: 01/04/2020 to 31/05/2020
Funding: Mixed (Hospital and Social Welfare Organizations Administration Commission, Ministry of Health and Welfare, Taiwan biotec donation of investigational products)
Conflict of interest: No
Trial NCT04261517
Publication Chen J, Journal of Zhejiang (2020) (published paper)
Dates: 06feb2020 to 25feb2020
Funding: Public/non profit (Shanghai Public Health Clinical Center)
Conflict of interest: No
Trial ChiCTR2000030054
Publication Chen L, medRxiv (2020) (preprint)
Dates: 18feb2020 to 30mar2020
Funding: Public/non profit (Medical and Health Key project of Xiamen, a project of the Xiamen Science and Technology Bureau)
Conflict of interest: No
Trial ChiCTR2000029559
Publication Chen Z, medRxiv (2020) (preprint)
Dates: 04feb2020 to 28feb2020
Funding: Public/non profit (Epidemiological Study of COVID-19 Pneumonia to Science and Technology Department of Hubei Province)
Conflict of interest: No
Trial NCT04325893
Publication Dubee V, Clin Microbiol Infec (2021) (published paper)
Dates: 2020-04-01 to 2020-05-26
Funding: Public/non profit (French Ministry of Health, Pays de la Loire region, Angers Loire Metropole conurbation)
Conflict of interest: Yes
Trial CTRI/2020/04/024479
Publication Gupta S, Med J Armed Forces I (2021) (published paper)
Dates: 2020-05-30 to 2020-09-30
Funding: Public/non profit (Command Hospital Airforce)
Conflict of interest: No
Trial NCT04315896
Publication Hernandez-Cardenas C, PloS One (2021) (published paper)
Dates: 2020-04-08 to 2020-07-12
Funding: Mixed (Participating hospitals; CONACYT (National Council of Science and Technology of Mexico); SANOFI)
Conflict of interest: *
Trial NCT04381936
Publication RECOVERY (HCQ) - Horby P, N Engl J Med (2020) (published paper)
Dates: 25mar2020 to 05jun2020
Funding: Mixed (University of Oxford from UK Research and Innovation/National Institute for Health Research (NIHR); NIHR Oxford Biomedical Research Centre; Wellcome; Bill and Melinda Gates Foundation; Department for International Development; Health Data Research UK)
Conflict of interest: No
Trial NCT04491994
Publication Kamran SM, Cureus (2021) (published paper)
Dates: 2020-04-10 to 2020-05-17
Funding: No specific funding (No financial support was received from any organization for the submitted work. )
Conflict of interest: No
Trial NCT04316377
Publication NO COVID-19 - Lyngbakken MN, Nature (2020) (published paper)
Dates: 2020-03-25 to 2020-05-25
Funding: Public/non profit (University Hospital, Akershus;European Virus Archive Global (EVA-GLOBAL) project [publication])
Conflict of interest: No
Trial ISRCTN83971151; NCT04315948
Publication SOLIDARITY (HCQ) - Pan H, N Engl J Med (2020) (published paper)
Dates: 2020-03-22 to 2020-10-04
Funding: Mixed (World Health Organization; Mylan (drug donation))
Conflict of interest: No
Trial NCT04332991
Publication ORCHID - Self W, JAMA (2020) (published paper)
Dates: 2020-04-02 to 2020-06-19
Funding: Mixed (National Heart, Lung, and Blood Institute (NHLBI), NCATS, Harvard Catalyst, Sandoz.)
Conflict of interest: Yes
Trial ChiCTR2000029868
Publication Tang W, BMJ (2020) (published paper)
Dates: 11feb2020 to 29feb2020
Funding: Mixed (Emergent Projects of National Science and Technology; National Natural Science Foundation of China; National Key Research and Development Program of China; Shanghai Municipal Key Clinical Specialty; National Innovative Research Team of High-level Loc)
Conflict of interest: No
Trial NCT04369742
Publication TEACH - Ulrich RJ, Open Forum Infect Di (2020) (published paper)
Dates: 2020-04-17 to 2020-05-12
Funding: Public/non profit (NYU Grossman School of Medicine; National Center for Advancing Translational Sciences, National Institutes of Health)
Conflict of interest: No