Hydroxychloroquine + Azithromycin vs Hydroxychloroquine (RCT)
Mild outpatients
FOREST PLOTS -2022-04-12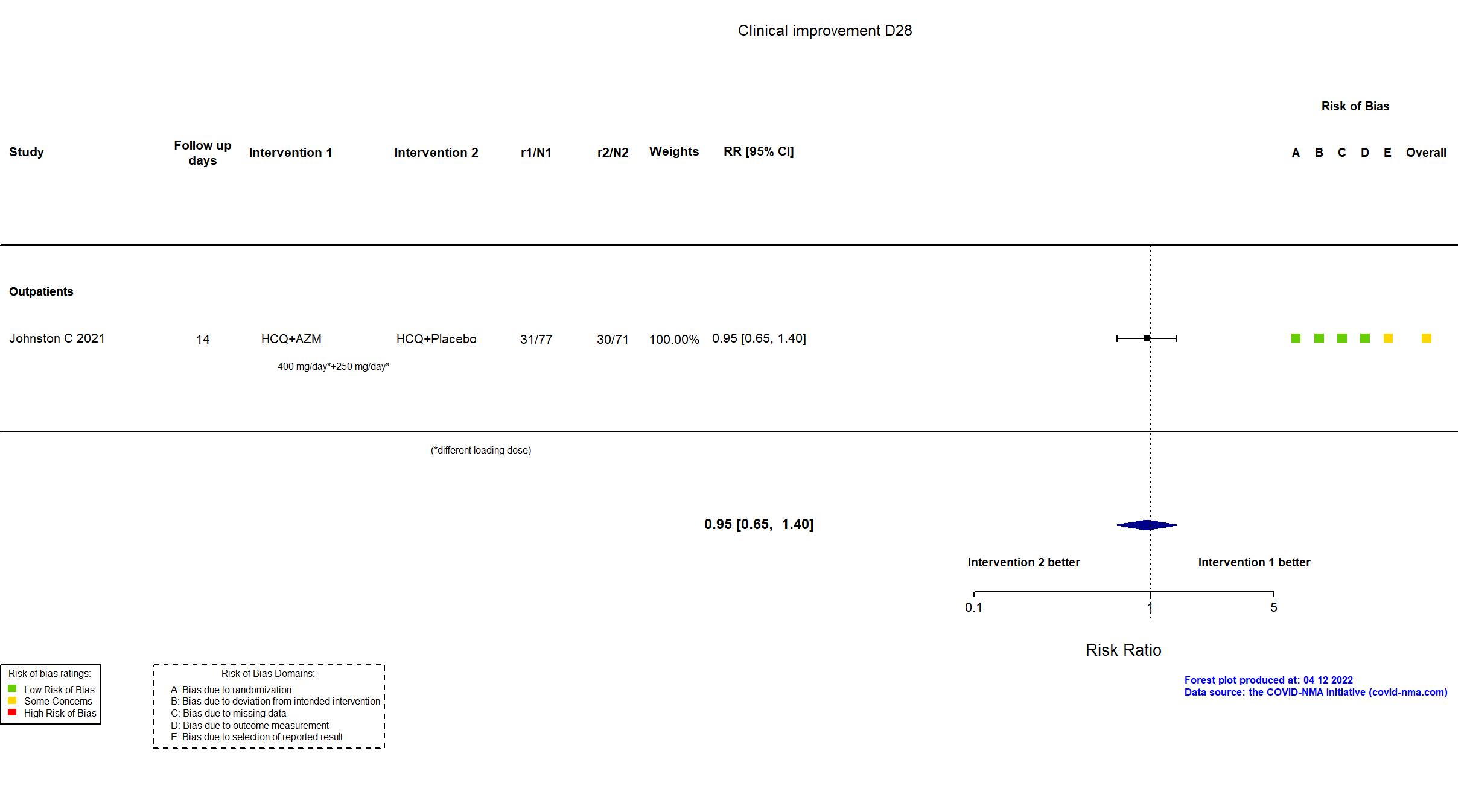
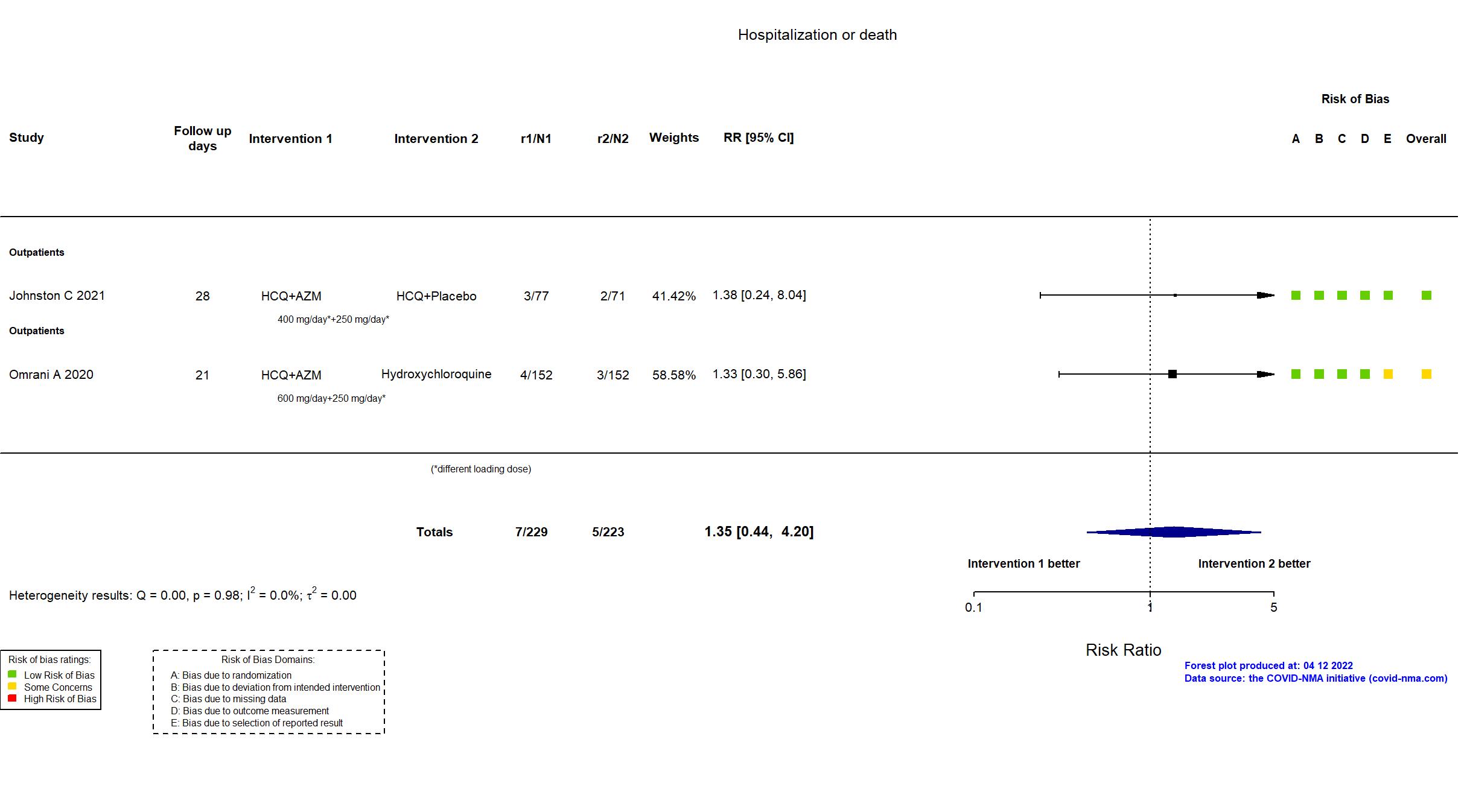

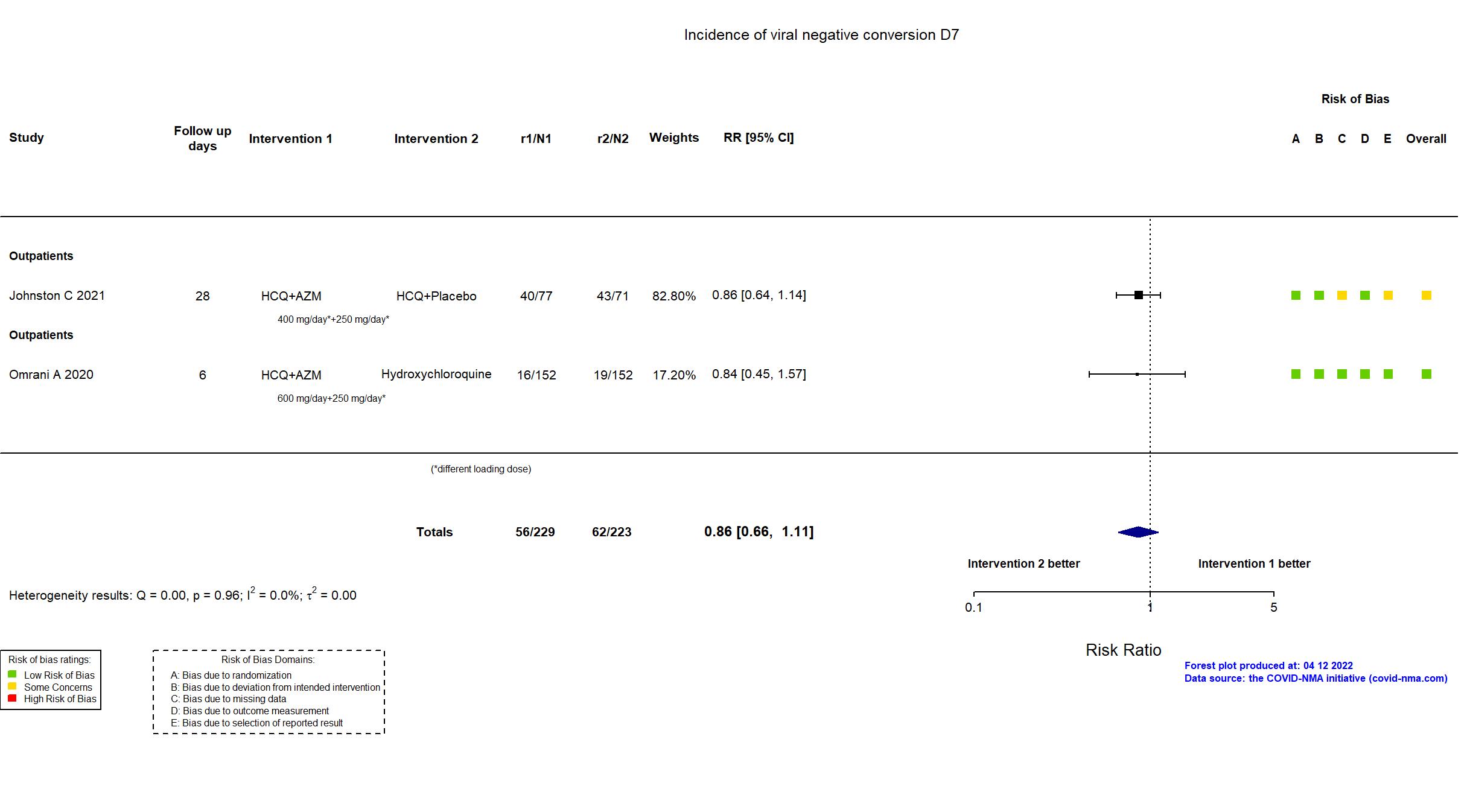
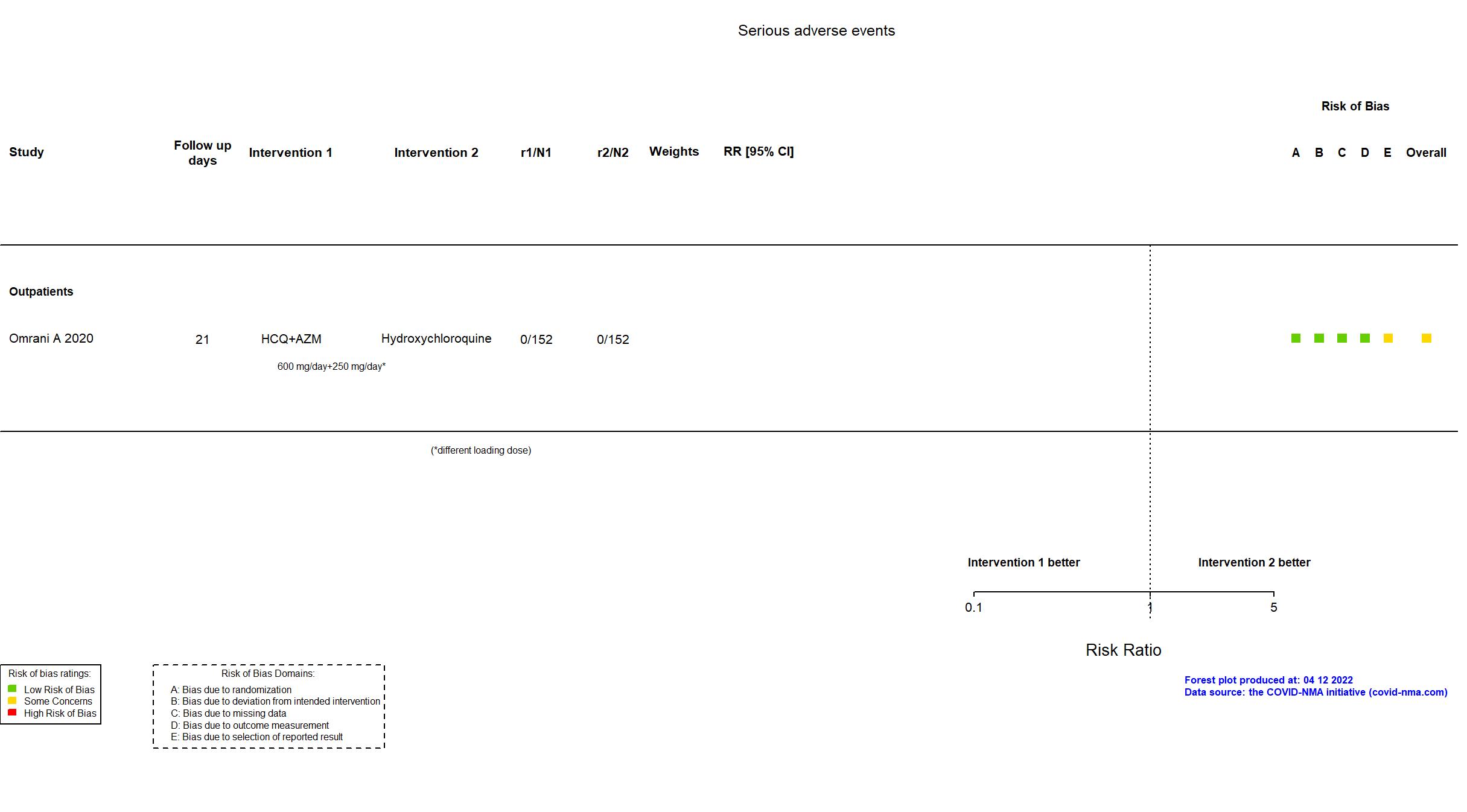





Trial NCT04354428
Publication Johnston C, EClinicalMedicine (2021) (published paper)
Dates: 2020-04-15 to 2020-07-27
Funding: Public/non profit (Bill & Melinda Gates Foundation; University of Washington Institute of Translational Health Science (ITHS) grant support from NCATS/NIH funded REDCap)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Participants, outcome assessor and health care pro | |
| Location :
Multicenter / USA Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
HCQ+Placebo HCQ: Initial dose: 400 mg orally twice daily on Day 1. Maintenance dose: 200 mg orally twice daily for 9 days. Placebo (folic acid) Initial dose: 800 mcg orally once daily on Day 1. Maintenance dose: 400 mcg orally once daily for 4 days. HCQ+AZM HCQ: Initial dose: 400 mg orally twice daily on Day 1. Maintenance dose: 200 mg orally twice daily for 9 days. AZM: Initial dose: 500 mg orally once daily on Day 1. Maintenance dose: 250 mg orally once daily for 4 days. |
|
| Control
Vitamin C+Placebo Vitamin C: Initial dose: 500 mg orally twice daily on Day 1. Maintenance dose: 250 mg orally twice daily for 9 days. Placebo (folic acid) Initial dose: 800 mcg orally once daily on Day 1. Maintenance dose: 400 mcg orally once daily for 4 days. | |
| Participants | |
| Randomized participants : Vitamin C+Placebo=83 HCQ+Placebo=71 HCQ+AZM=77 | |
| Characteristics of participants N= 231 Mean age : NR 100 males Severity : Mild: n= 212/ Asymptomatic: n=19 | |
| Primary outcome | |
| In the register Lower respiratory tract infection (LRTI) rates [ Time Frame: 28 days from enrolment ] Resting blood oxygen saturation (SpO2<93%) level sustained for 2 readings 2 hours apart and presence of subjective dyspnea or cough Incidence of hospitalization or mortality [ Time Frame: Day 28 after enrolment ] Cumulative incidence of hospitalization or mortality Change in upper respiratory viral shedding [ Time Frame: Day 1 through Day 14 after enrolment ] Time to clearance of nasal SARS-CoV-2, defined as 2 consecutive negative swabs | |
| In the report Development of LRTI, defined by SpO2<93% on two readings ≥two hours but | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the study registry, protocol, and statistical analysis plan were used in data extraction and risk of bias assessment. The article reports two treatment arms plus a control arm of a platform study assessing several treatments. Due to the low rate of clinical outcomes, the study was terminated for operational futility. As a result, the target sample size specified in the registry was not achieved. As the medication had a distinctive taste, to maintain participant blinding, all members of a household were randomized to the same treatment group. There is no change from the trial registration in the intervention and control treatments. |
Trial NCT04349592
Publication Q-PROTECT - Omrani A, EClinicalMedicine (2020) (published paper)
Dates: 2020-04-13 to 2020-08-01
Funding: Public/non profit (Hamad Medical Corporation (government health service of the State of Qatar))
Conflict of interest: No
| Methods | |
| RCT Blinding: | |
| Location :
Multicenter / Qatar Follow-up duration (days): 21 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
HCQ+AZM Hydroxychloroquine: 200 mg orally 3 times a day for 7 days. Azithromycin: 500 mg orally on day 1 followed by 250 mg orally once a day for the next 4 days. Hydroxychloroquine 200 mg orally 3 times a day for 7 days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : HCQ+AZM=152 Hydroxychloroquine=152 Placebo=152 | |
| Characteristics of participants N= 456 Mean age : NR 449 males Severity : Mild: n= 456/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register Proportion of virologically cured (PCR-negative status) as assessed on day six [ Time Frame: Day 6 ] | |
| In the report Virologic cure (PCR-negative status) as assessed on day six. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan NR Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the trial registry and study protocol were used for data extraction and assessment of risk of bias. No statistical analysis plan was available. There were no substantive differences between the published article, the current trial registration and the study protocol in terms of procedures, population or treatments. The trial registry was changed near to end of recruitment to include outcomes not included in the original entry and the study protocol is not dated, but the data extracted are not affected. The study achieved its pre-stated sample size. Although open to both sexes and conducted in Qatar, the study population was overwhelmingly male and no Qataris were included, reflecting the country’s workforce. |