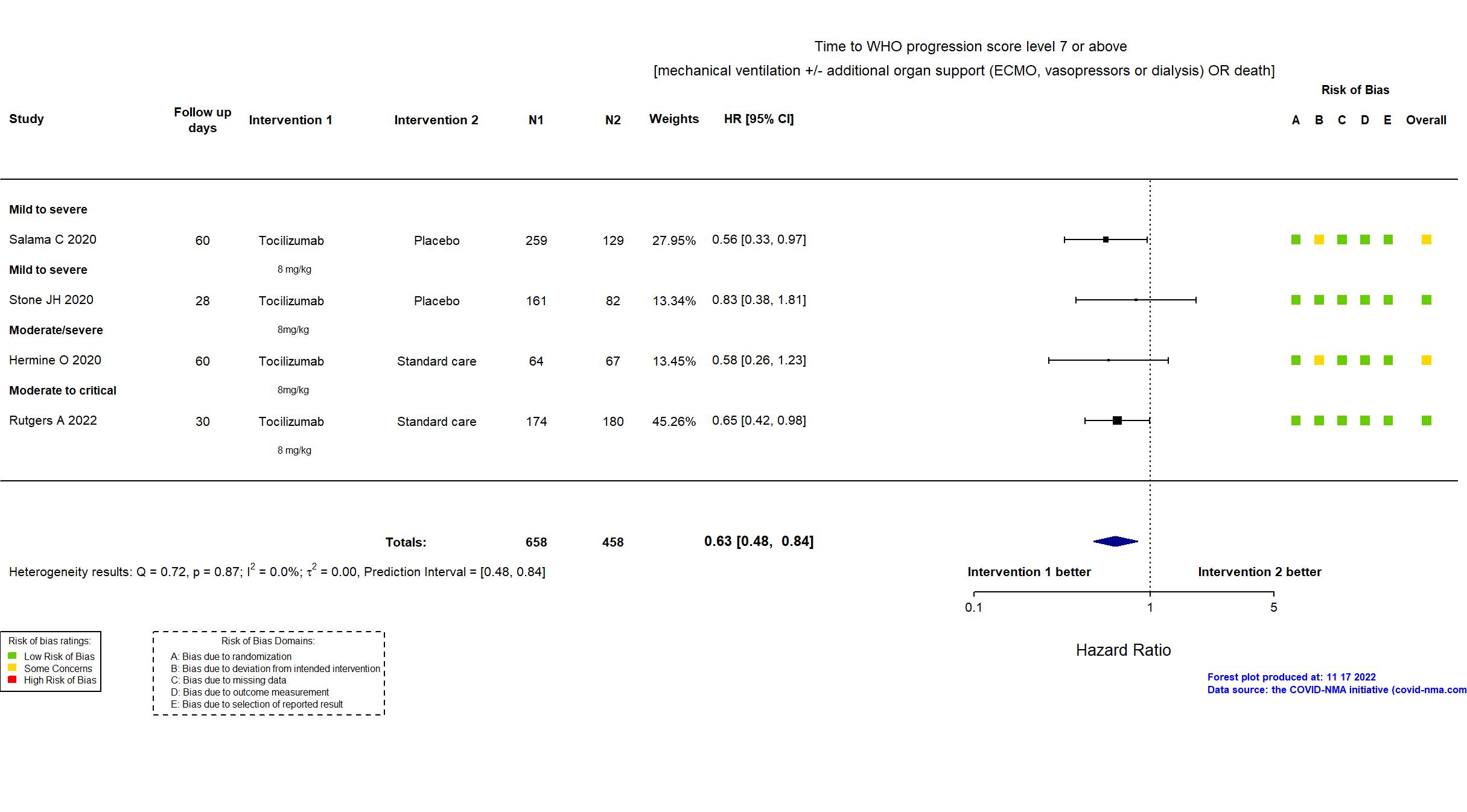
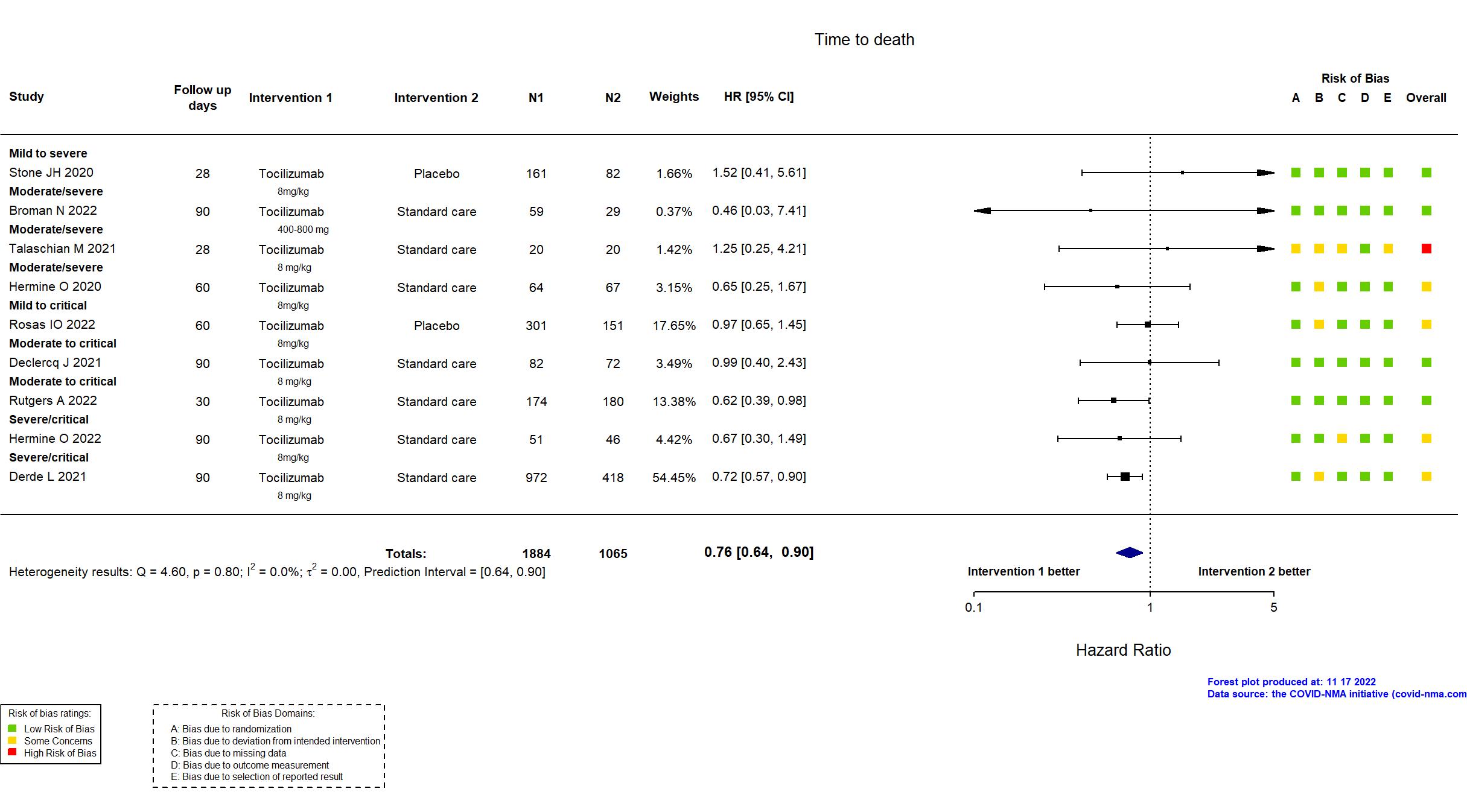
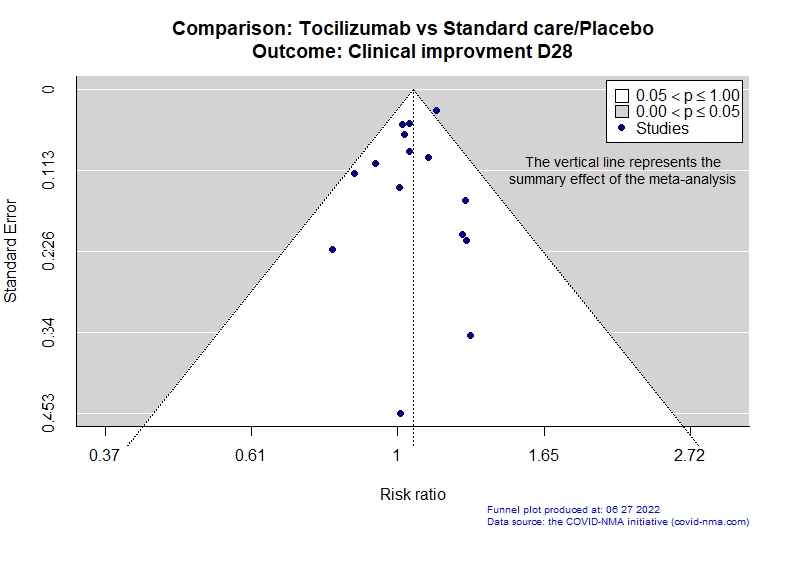
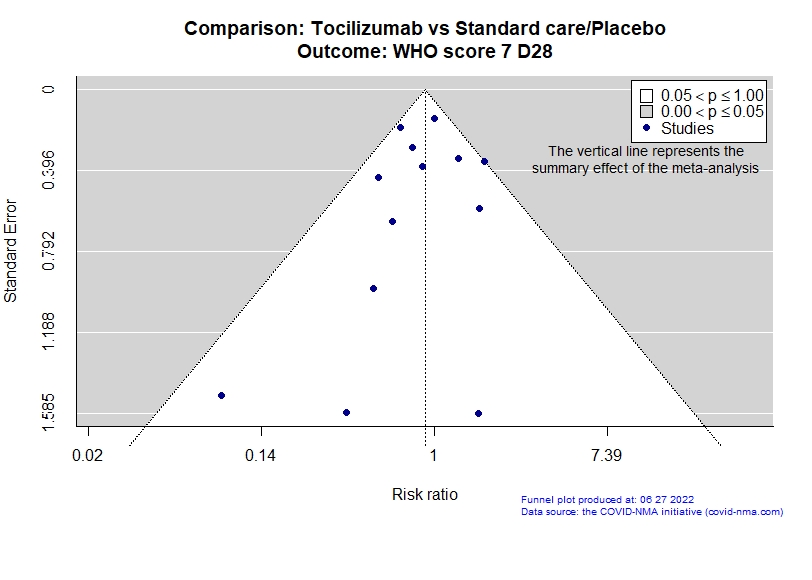
Studies description
Trial NCT04412772
Publication ARCHITECTS - ARCHITECTS, Unpublished (2021) (unpublished results)
Dates: 2020-06-12 to 2020-08-28
Funding: Public/non profit (Queen's Medical Centre)
Conflict of interest: *
Trial NCT04577534
Publication COVIDSTORM - Broman N, Clin Microbiol Infect (2022) (published paper)
Dates: 2020-08-12 to 2021-06-16
Funding: No specific funding (No external funding was received
for this study or article.)
Conflict of interest: Yes
Trial NCT04479358
Publication COVIDOSE-2 - COVIDOSE-2, Unpublished (2021) (unpublished results)
Dates: 2020-09-10 to 2021-01-31
Funding: Public/non profit (University of Chicago )
Conflict of interest: *
Trial NCT04435717
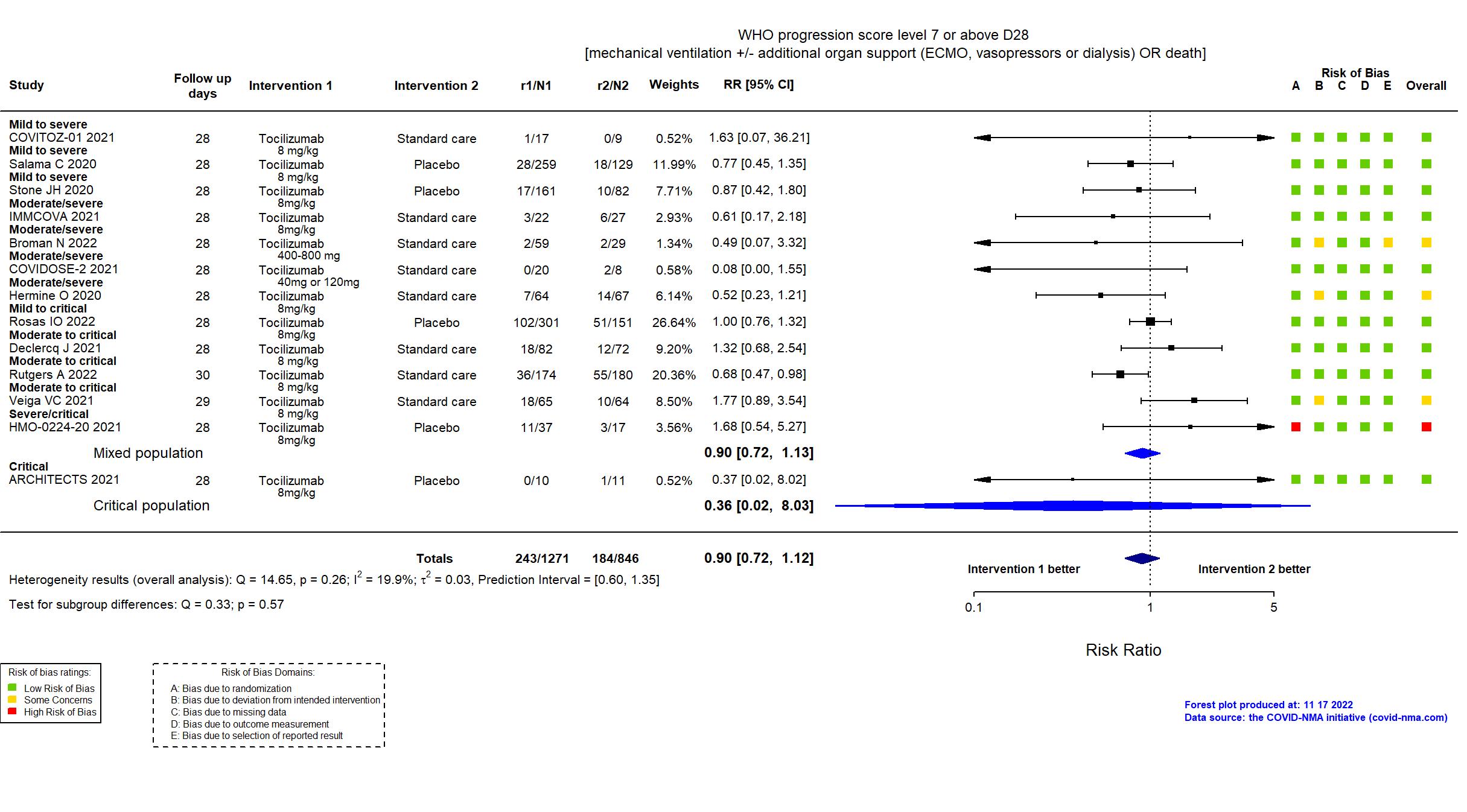
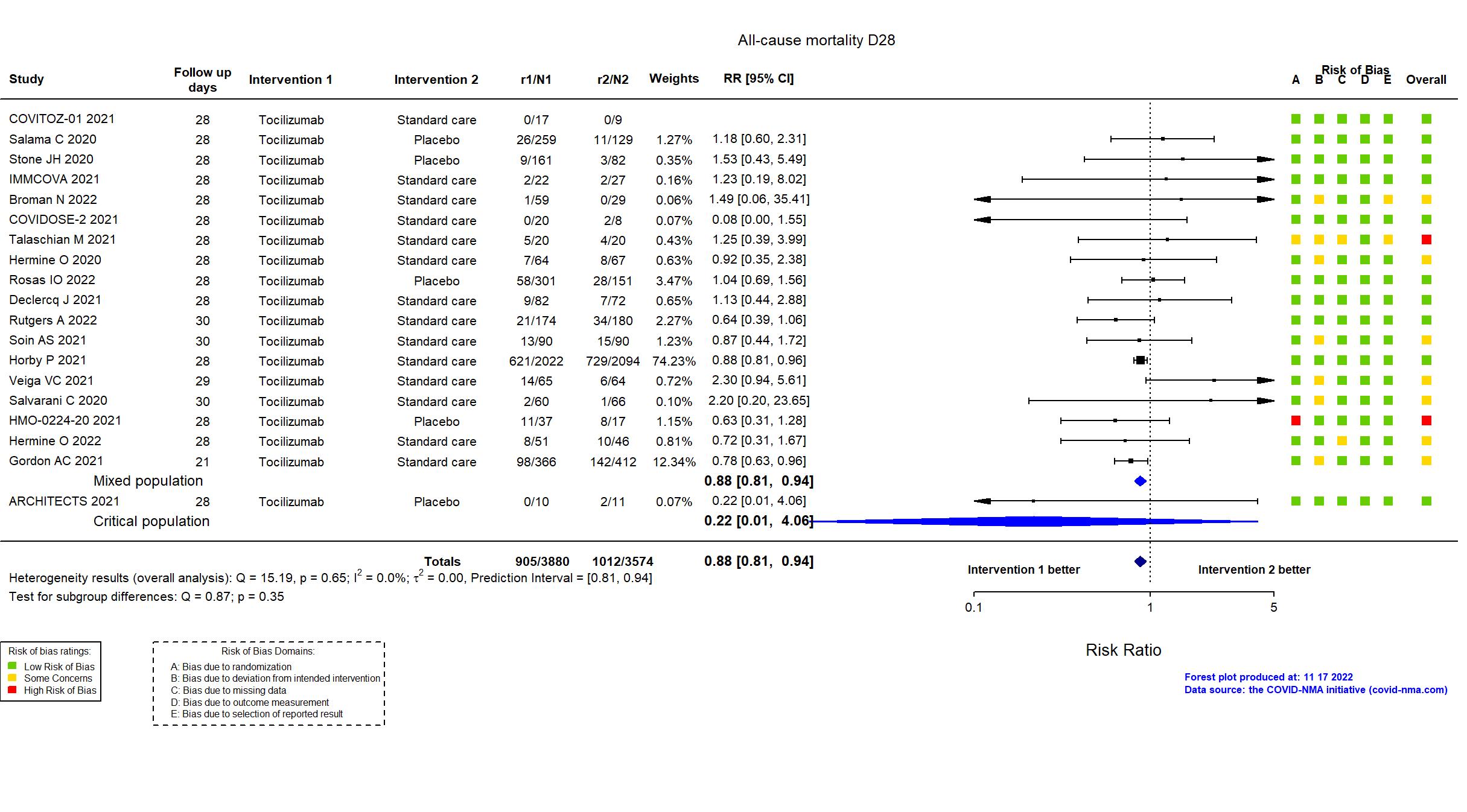
Publication COVITOZ-01 - COVITOZ-01, Unpublished (2021) (unpublished results)
Dates: 2020-05-04 to 2020-10-21
Funding: Public/non profit (Hospital Universitario Ramon y Cajal)
Conflict of interest: *
Trial NCT04330638; EudraCT2020-001500-41
Publication COV-AID - Declercq J, Lancet Respir Med (2021) (published paper)
Dates: 2020-04-04 to 2020-12-06
Funding: Public/non profit (Belgian Health Care Knowledge Center; VIB Grand Challenges (Flemish Institute for Biotechnology))
Conflict of interest: No
Trial NCT02735707
Publication REMAP-CAP - Derde L, medRxiv (2021) (preprint)
Dates: 2020-03-25 to 2021-04-10
Funding: Mixed (PREPARE consortium by the European Union; FP7-HEALTH-2013-INNOVATION-1; RECOVER consortium by the European Union Horizon 2020 research and innovation program; Australian National Health and Medical Research Council; Health Research Council of New Zealand; Canadian Institute of Health Research Strategy for Patient-Oriented Research Innovative Clinical Trials Program Grant; UK NIHR; NIHR Imperial Biomedical Research Centre; Health Research Board of Ireland; UPMC Learning While Doing Program; Translational Breast Cancer Research Consortium; Global Coalition for Adaptive Research; French Ministry of Health; Minderoo Foundation; Wellcome Trust Innovations Project; Netherlands Organization for Health Research and Development ZonMw; NIHR Research Professorship; NIHR Clinician Scientist Fellowship; Australian National Health and Medical Research Council Career Development Fellowship; Roche Products Ltd; Sanofi (Aventis Pharma Ltd); Swedish Orphan Biovitrum AB (Sobi); Faron Pharmaceuticals (drug provision in some countries)
)
Conflict of interest: No
Trial NCT02735707
Publication REMAP-CAP - Gordon AC, N Engl J Med (2021) (published paper)
Dates: 2020-04-19 to 2020-11-19
Funding: Mixed (Multiple funders, internationally, with multiple regional sponsors; Roche
Products Ltd and Sanofi (drug provision in UK))
Conflict of interest: Yes
Trial NCT04331808
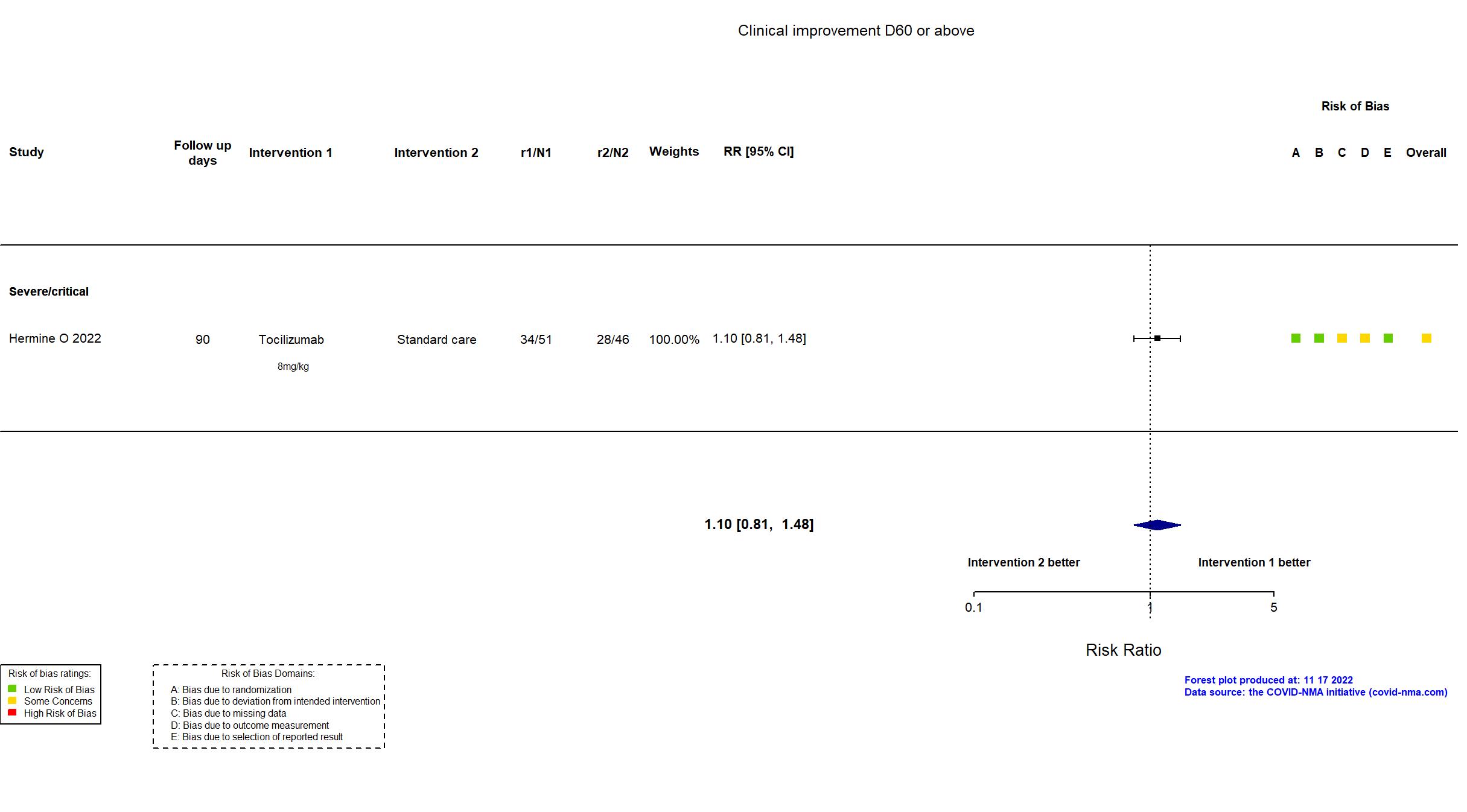
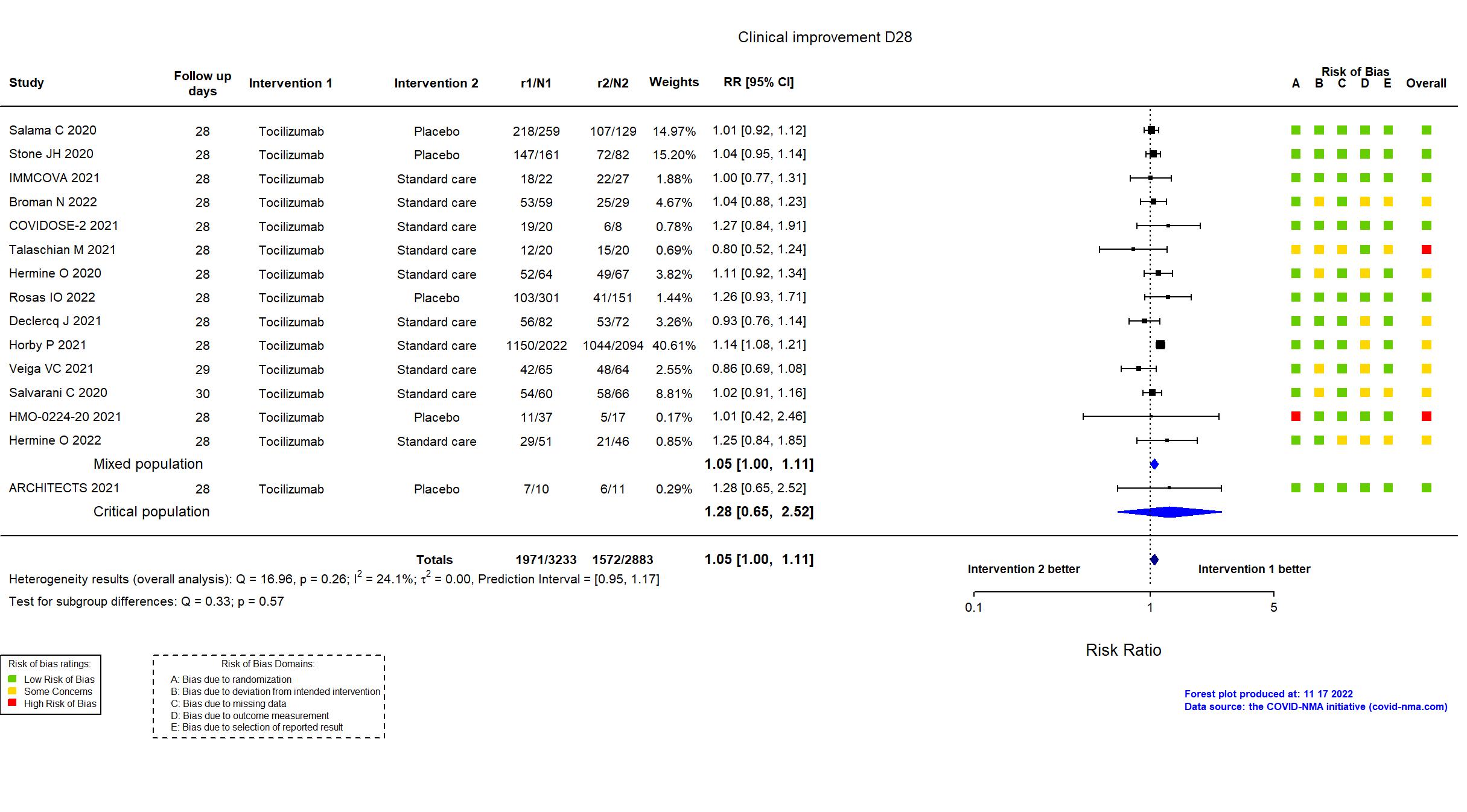
Publication CORIMUNO-TOCI-2 - Hermine O, Eur Respir J (2022) (published paper)
Dates: 2020-03-30 to 2020-04-20
Funding: Public/non profit (Assistance Publique - Hôpitaux de Paris)
Conflict of interest: No
Trial NCT04331808
Publication CORIMUNO-TOCI 1 - Hermine O, JAMA Intern Med (2020) (published paper)
Dates: 2020-03-31 to 2020-04-18
Funding: Public/non profit (This trial was publicly funded (Ministry of Health, Programme Hospitalier de Recherche Clinique, Foundation for Medical Research (FRM), AP-HP Foundation and the Reacting program).)
Conflict of interest: No
Trial NCT04377750
Publication HMO-0224-20 - HMO-0224-20, Unpublished (2021) (unpublished results)
Dates: 2020-04-08 to 2021-02-03
Funding: Public/non profit (Hadassah Medical Organization)
Conflict of interest: *
Trial NCT04381936, ISRCTN50189673
Publication RECOVERY (TCZ) - Horby P, Lancet (2021) (published paper)
Dates: 2020-04-23 to 2021-01-24
Funding: Public/non profit (UK research and Innovation/National Institute for Health Research (NIHR); NIHR Oxford Biomedical Research Centre, Wellcome; Bill and Melinda Gates Foundation; Department for International Development; Health Data Research UK; Medical Research Council Population Health Research Unit; NIHR Clinical Trials Unit Support Funding; Abbvie (lopinavir-ritonavir); Roche Products Ltd (tocilizumab); Regeneron (REGEN-480 COV2))
Conflict of interest: No
Trial NCT04412291, EudraCT 2020-001748-24
Publication IMMCOVA - IMMCOVA, Unpublished (2021) (unpublished results)
Funding: Public/non profit (Karolinska University Hospital)
Conflict of interest: *
Trial NCT04320615
Publication COVACTA - Rosas IO, EClinicalMedicine (2022) (published paper)
Dates: 2020-04-03 to 2020-05-28
Funding: Mixed (F. Hoffmann-La Roche Ltd; Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority)
Conflict of interest: Yes
Trial Trial NL8504
Publication Rutgers A, PLoS ONE (2022) (published paper)
Dates: 2020-04-06 to 2021-01-12
Funding: Mixed (Participating hospitals; Roche (drug supplier))
Conflict of interest: No
Trial NCT04372186
Publication EMPACTA - Salama C, N Engl J Med (2020) (published paper)
Dates: 2020-05-14 to 2020-08-18
Funding: Private (Genentech, Inc.)
Conflict of interest: Yes
Trial NCT04346355
Publication Salvarani C, JAMA (2020) (published paper)
Dates: 2020-03-31 to 2020-06-11
Funding: Mixed (Local resources, the Italian Ministry of Health and Roche)
Conflict of interest: Yes
Trial CTRI/2020/05/025369
Publication COVINTOC - Soin AS, Lancet Respir Med (2021) (published paper)
Dates: 2020-05-30 to 2020-08-31
Funding: Mixed (Medanta Institute of Education and Research; Roche India; Cipla India; Action COVID-19 India)
Conflict of interest: Yes
Trial NCT04356937
Publication Stone JH, N Engl J Med (2020) (published paper)
Dates: 2020-04-20 to 2020-06-15
Funding: Private (Genentech)
Conflict of interest: Yes
Trial IRCT20081027001411N4
Publication Talaschian M, Research Square (2021) (preprint)
Dates: 2020-07-10 to 2020-10-10
Funding: Public/non profit (Tehran University of Medical Sciences)
Conflict of interest: No
Trial NCT04403685
Publication TOCIBRAS - Veiga VC, BMJ (2021) (published paper)
Dates: 2020-05-08 to 2020-07-17
Funding: Mixed (The hospitals and research institutes participating in Coalition covid-19 Brazil; Fleury Laboratory (laboratory analysis); Instituto Votorantim (donation for drug provision))
Conflict of interest: Yes
Trial ChiCTR2000029765
Publication Wang D, Front Med (2021) (published paper)
Dates: 2020-02-13 to 2020-03-13
Funding: Public/non profit (Department of Science and Technology of Anhui Province and Health Commission of Anhui Province; China National Center for Biotechnology Development)
Conflict of interest: No