Interferon alpha-2b + Interferon gamma+ vs Interferon alpha-2b (RCT)
Hospitalized patients
FOREST PLOTS -2021-02-26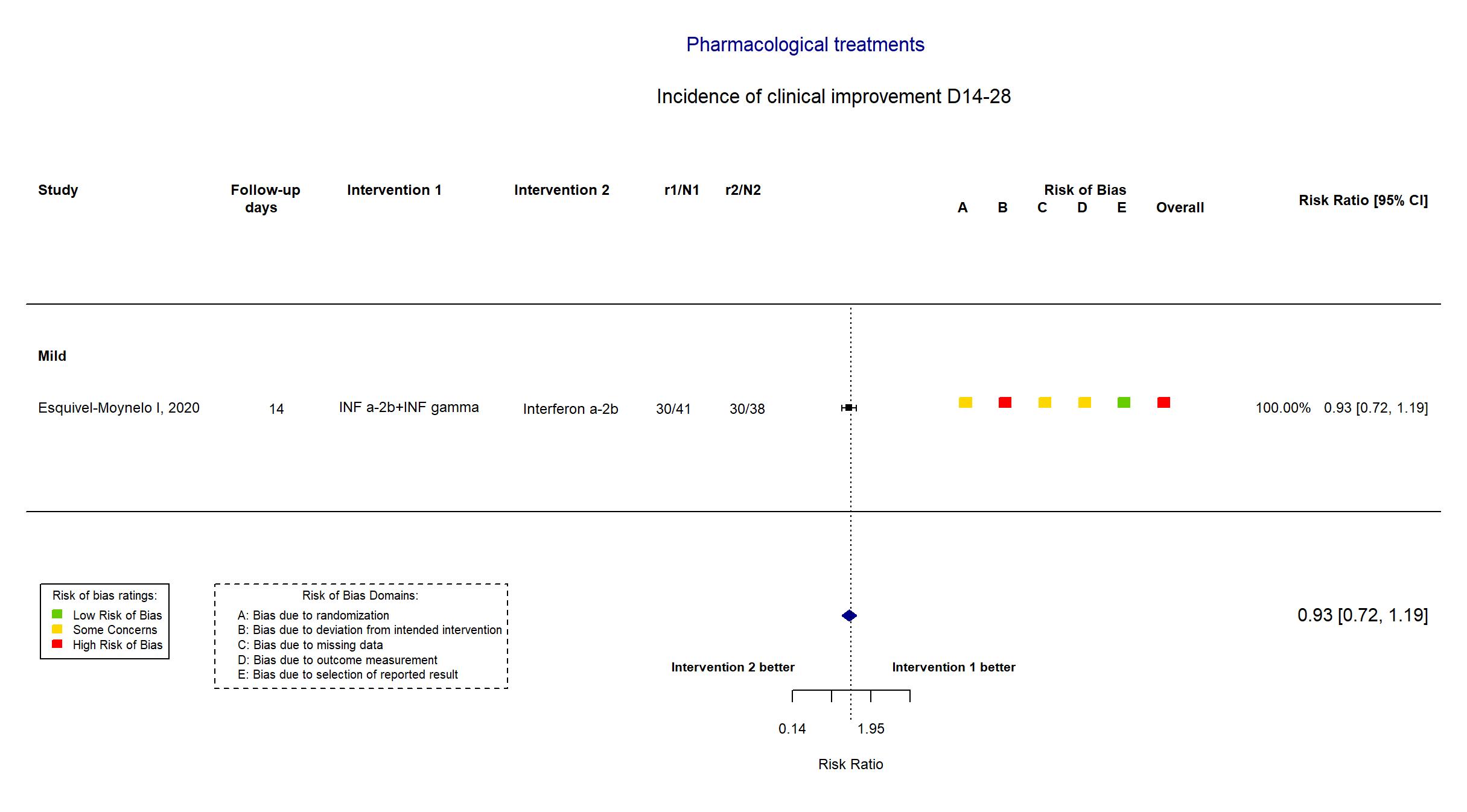
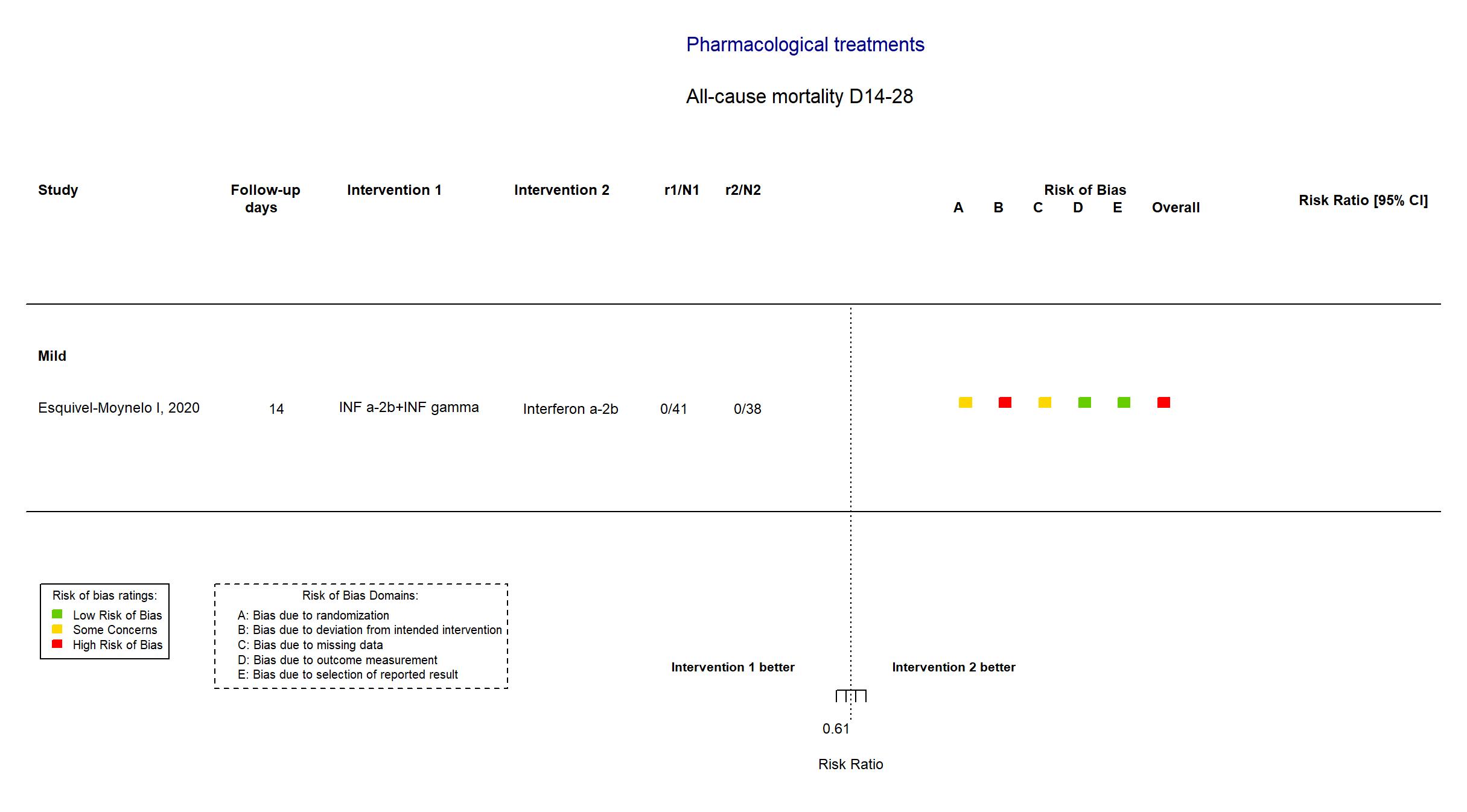
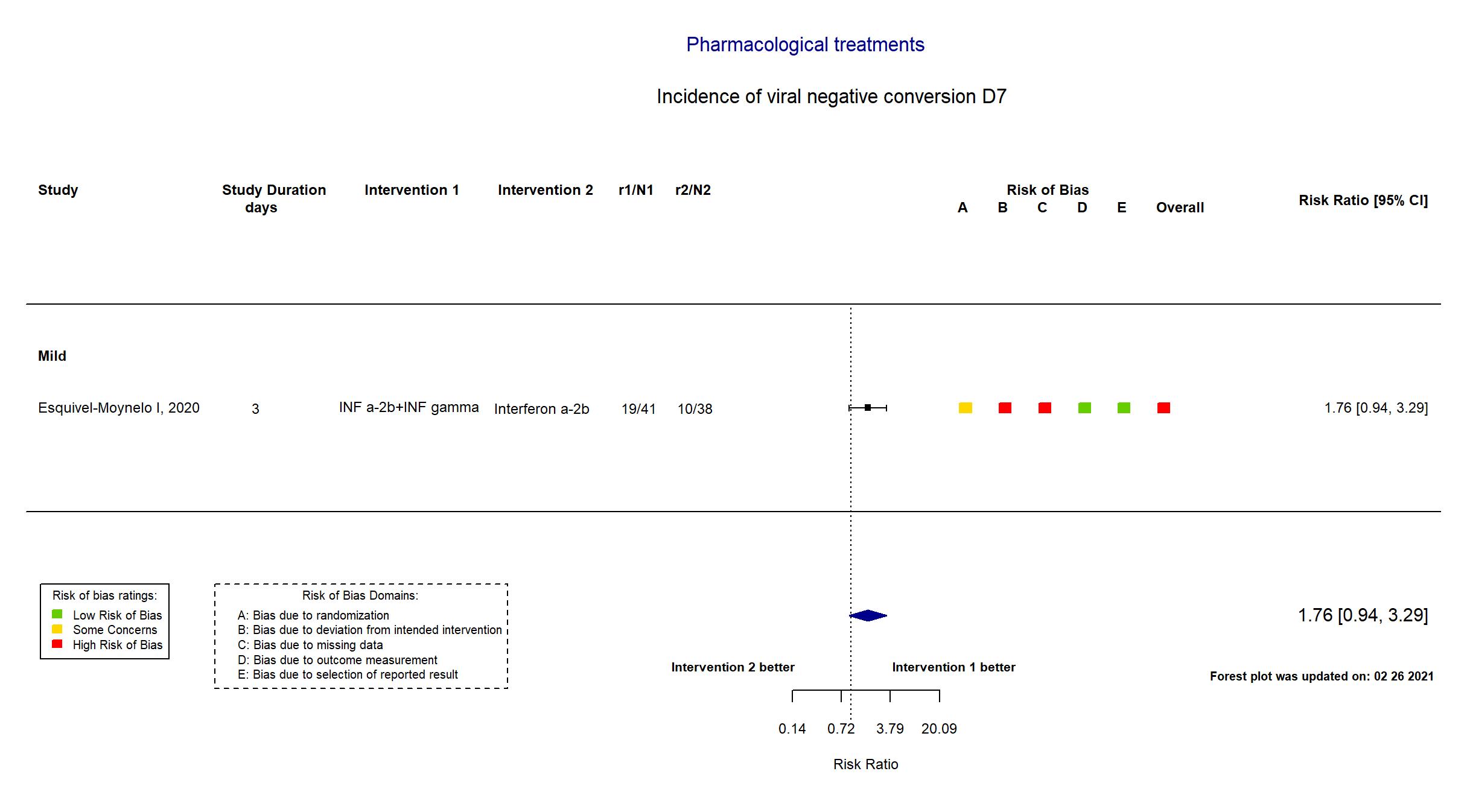
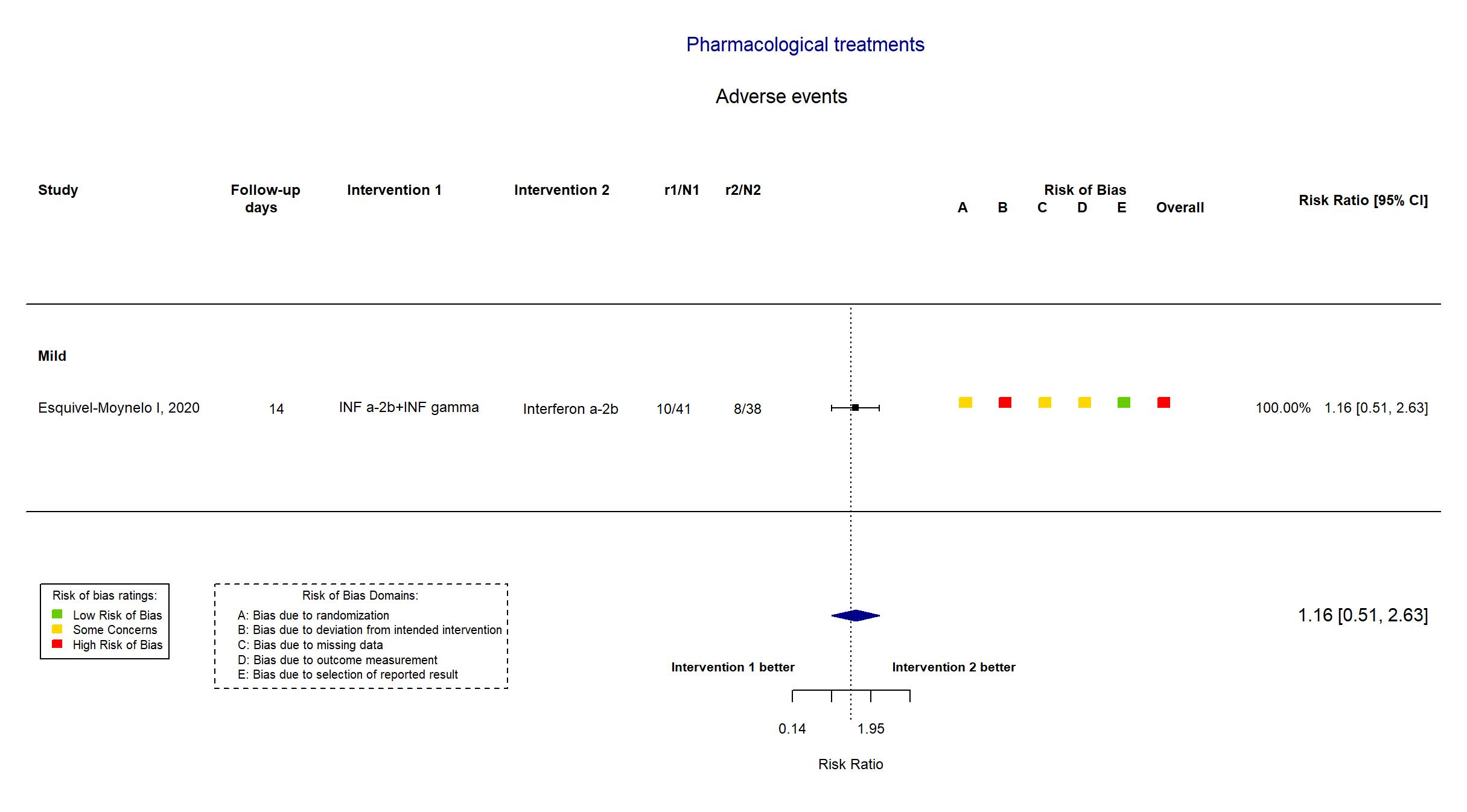






Trial RPCEC00000307
Publication Esquivel-Moynelo I, medRxiv (2020) (preprint)
Funding: Public/non profit (Center for Genetic Engineering and Biotechnology and Ministry of Health of Cuba.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / Cuba Follow-up duration (days): 14 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
INF a-2b+INF gamma IFN alpha2b: 3 MIU IFN gamma: 0.5 MIU Subcutaneous injection twice a week for 2 weeks |
|
| Control
Interferon alpha-2b 3 MIU intramuscularly 3 times a week | |
| Participants | |
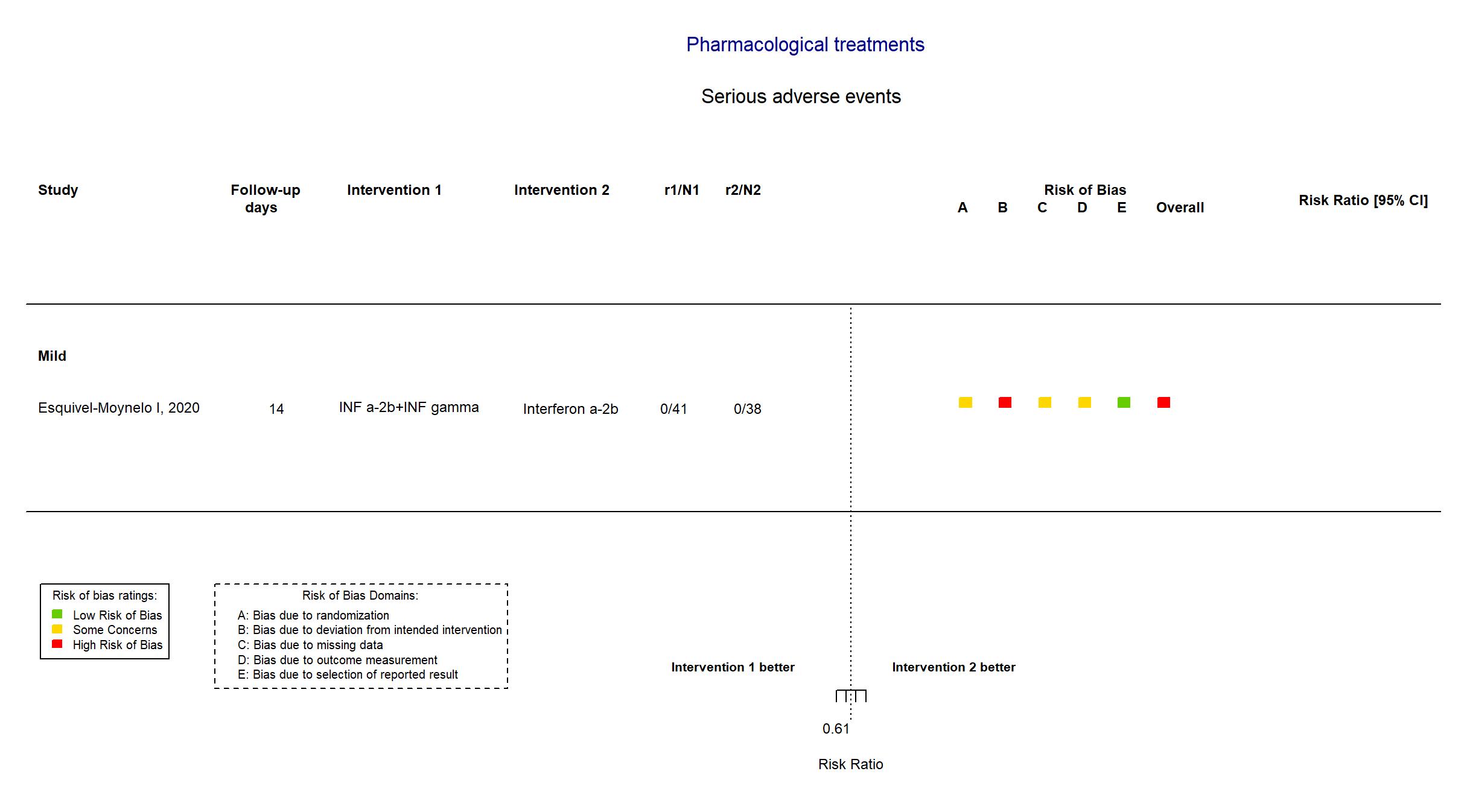
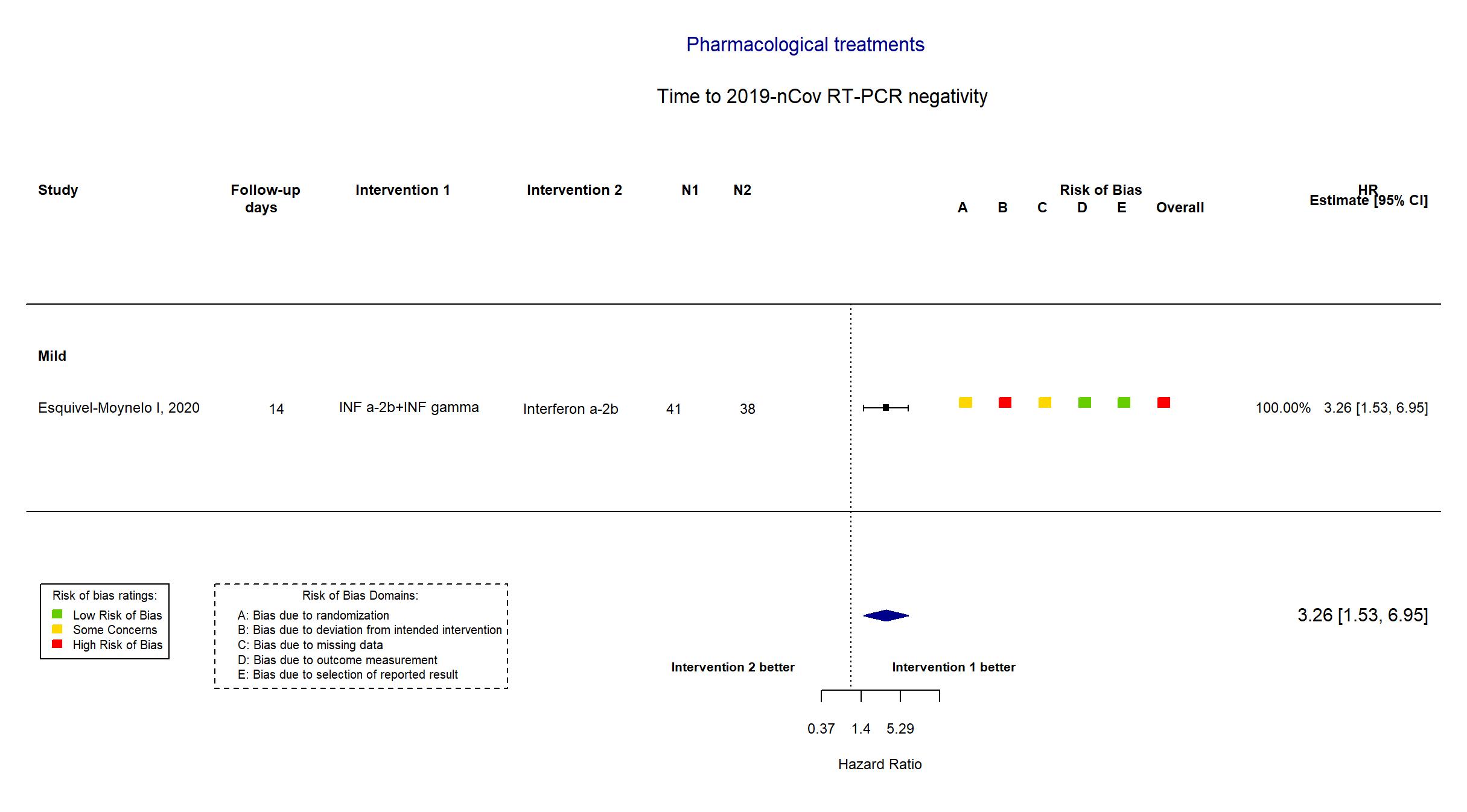
| Randomized participants : INF a-2b+INF gamma=41 Interferon alpha-2b=38 | |
| Characteristics of participants N= 79 Mean age : NR 34 males Severity : Mild: n=63 / Moderate: n=* / Severe: n=* Critical: n=* | |
| Primary outcome | |
| In the register Registro Publico Cubano de Ensayos Clinicos: In Spanish Quote from International Clinical Trials Registry Platform: | |
| In the report Time to SARS-CoV-2 RNA negativization (absence of the virus according to the RT-PCR) in positive patients after starting antiviral therapy was the virological endpoint. The clinical evaluation considered the time to progression to severe COVID-1 | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
High |
| General comment |
In addition to all available versions of the published article, the study registry, protocol, and statistical analysis plan were used in data extraction and risk of bias assessment. The study didn't achieve the target sample size specified in the trial registry (120). The trial registry was in Spanish but there were some discrepancies in study arm descriptions (duration, standard care definition) between the trial protocol and preprint. Quote from protocol: "The intervention group will receive the standard of care as well as, Kaletra and chloroquine as described, and HeberFERON. HeberFERON will be administered two times per week at 3.5 MIU for 3 weeks. The control group will receive standard of care as well as Heberon Alpha R, Kaletra, and chloroquine. Heberon Alpha R will be administered three times per week at 3.0 MIU for 3 weeks, with Kaletra and chloroquine". Quote from preprint: "Patients received 3.0 million international units (MIU) IFN-alpha2b and 0.5 MIU IFN-gamma (HeberFERON), twice a week for two weeks, subcutaneously and lopinavir-ritonavir 200/50 mg every 12 h and CQ 250 mg every 12 h (treatment group); or standard of care (3.0 MIU IFN-alpha2b (Heberon Alpha R), thrice a week, intramuscularly and lopinavir-ritonavir 200/50 mg every 12 h and CQ 250 mg every 12 h (control group)." Primary outcomes from the registry are reported in the paper, but not secondary outcomes. |