Interferon Beta vs Standard care/Placebo (RCT)
Hospitalized patients
FOREST PLOTS -2021-06-18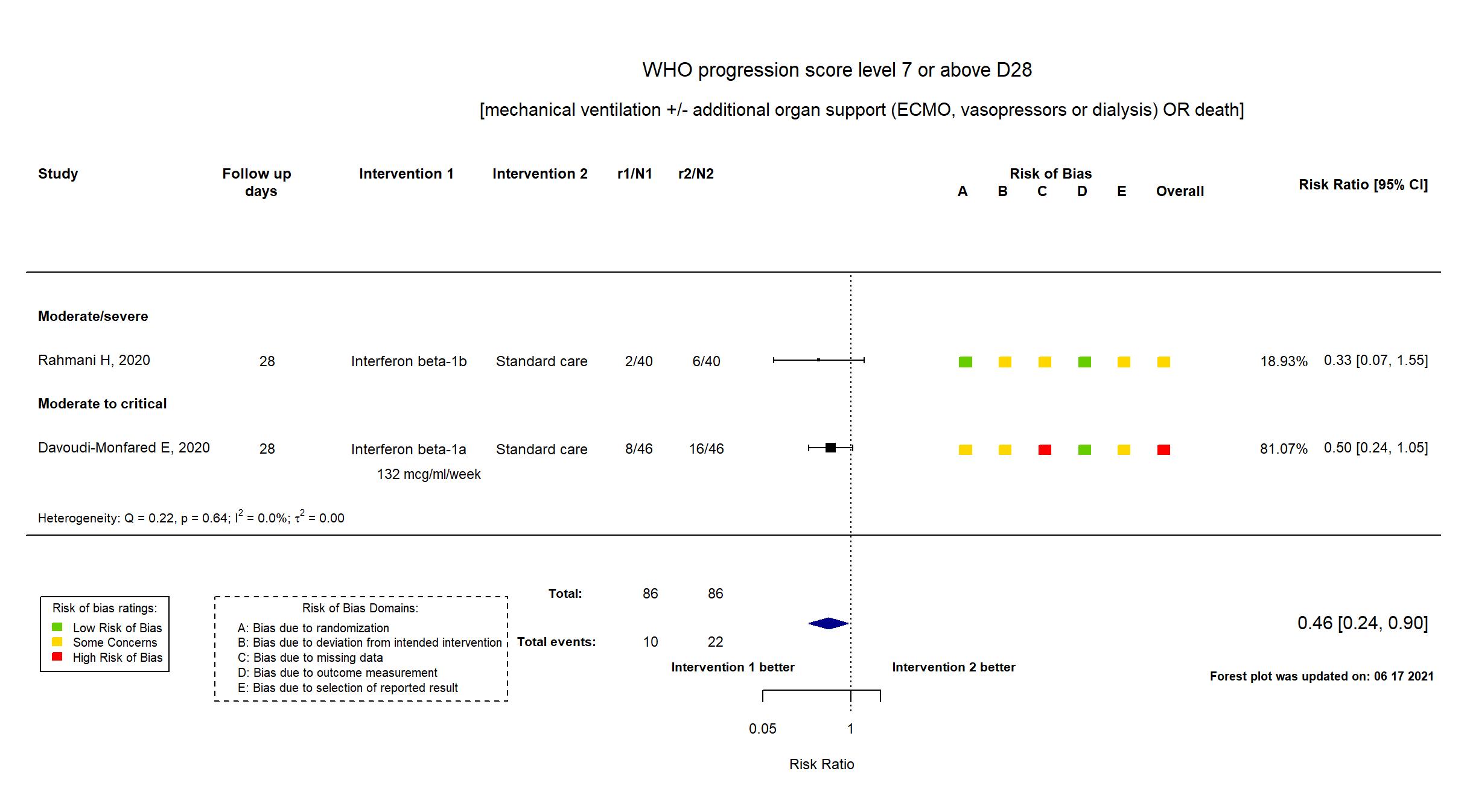
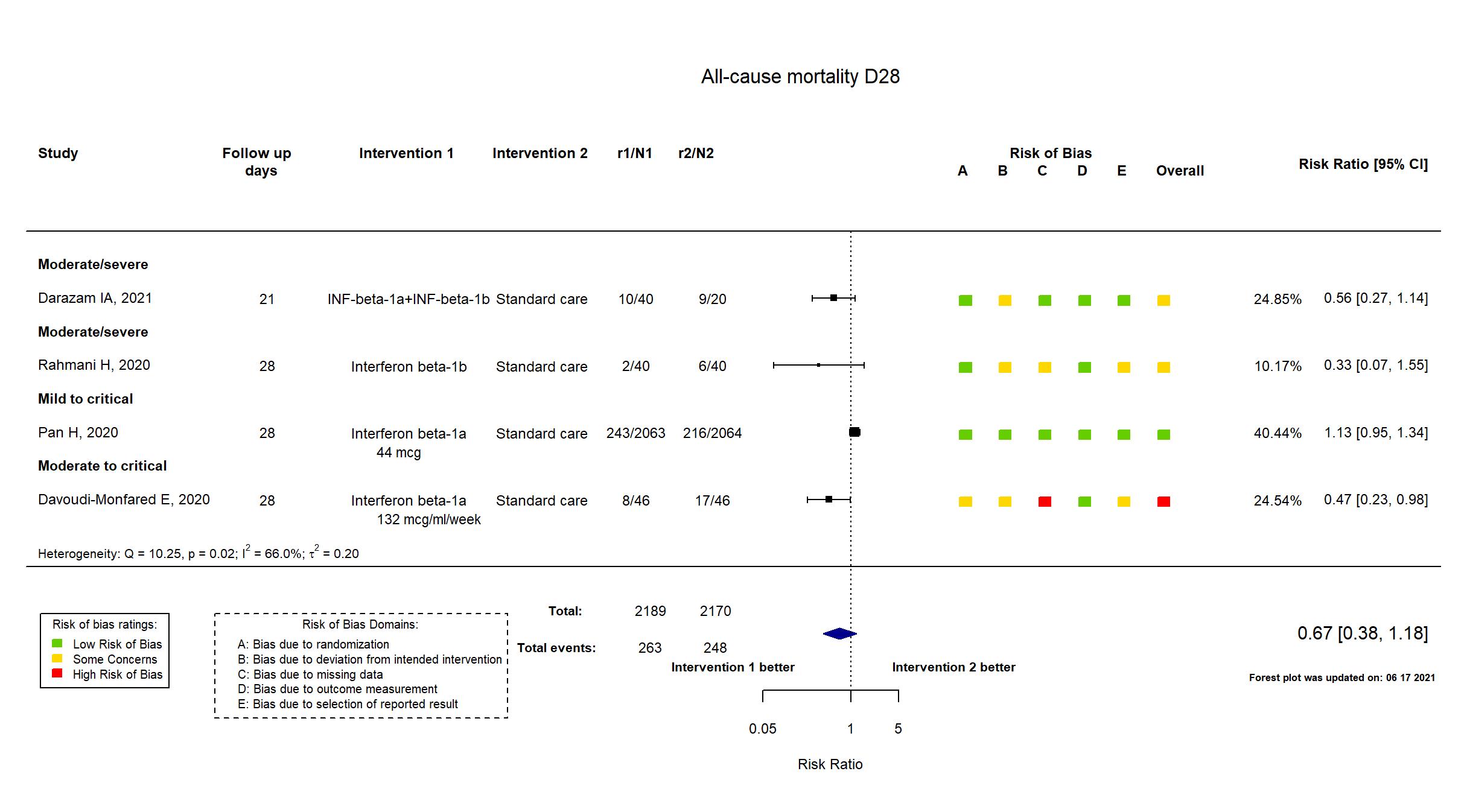
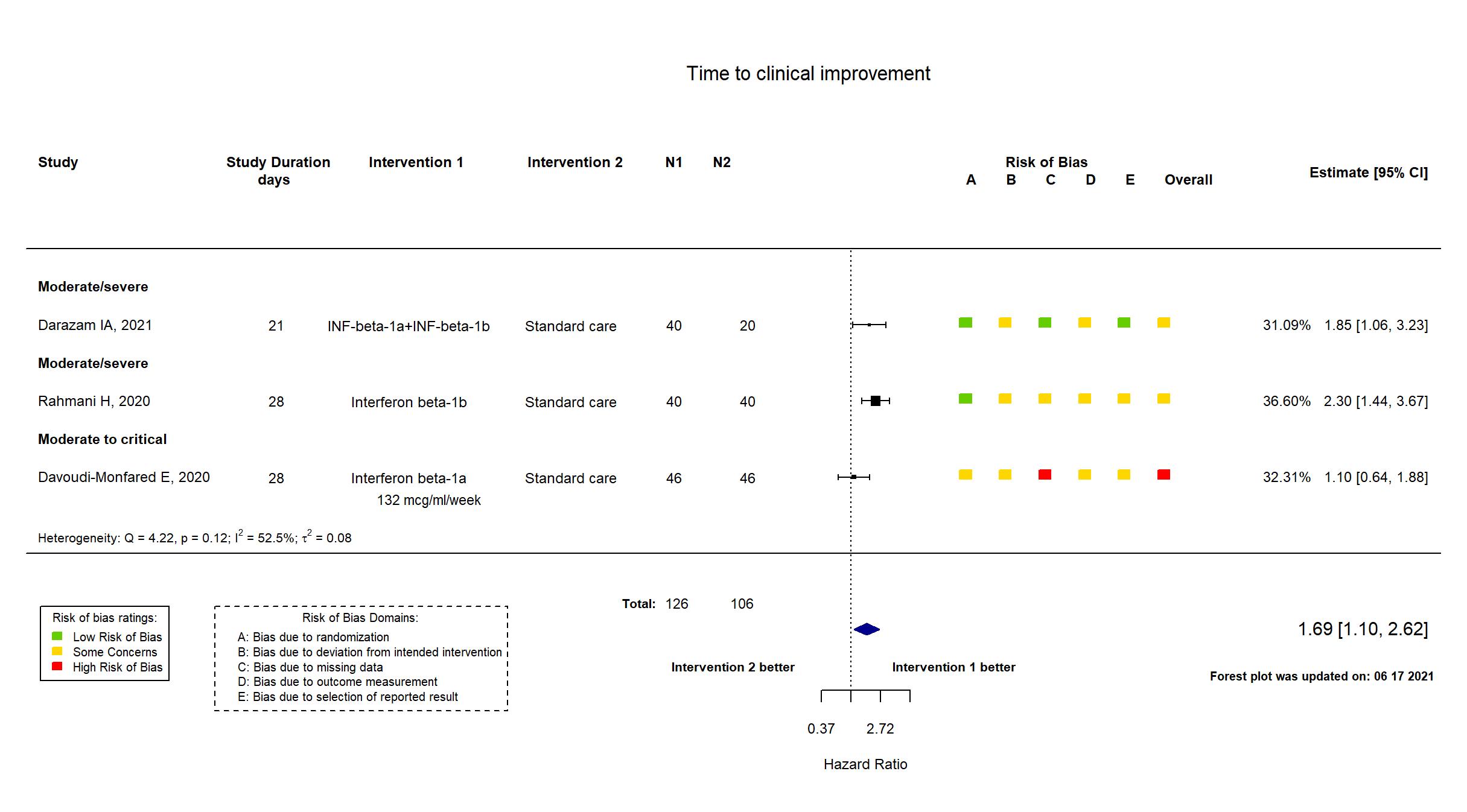
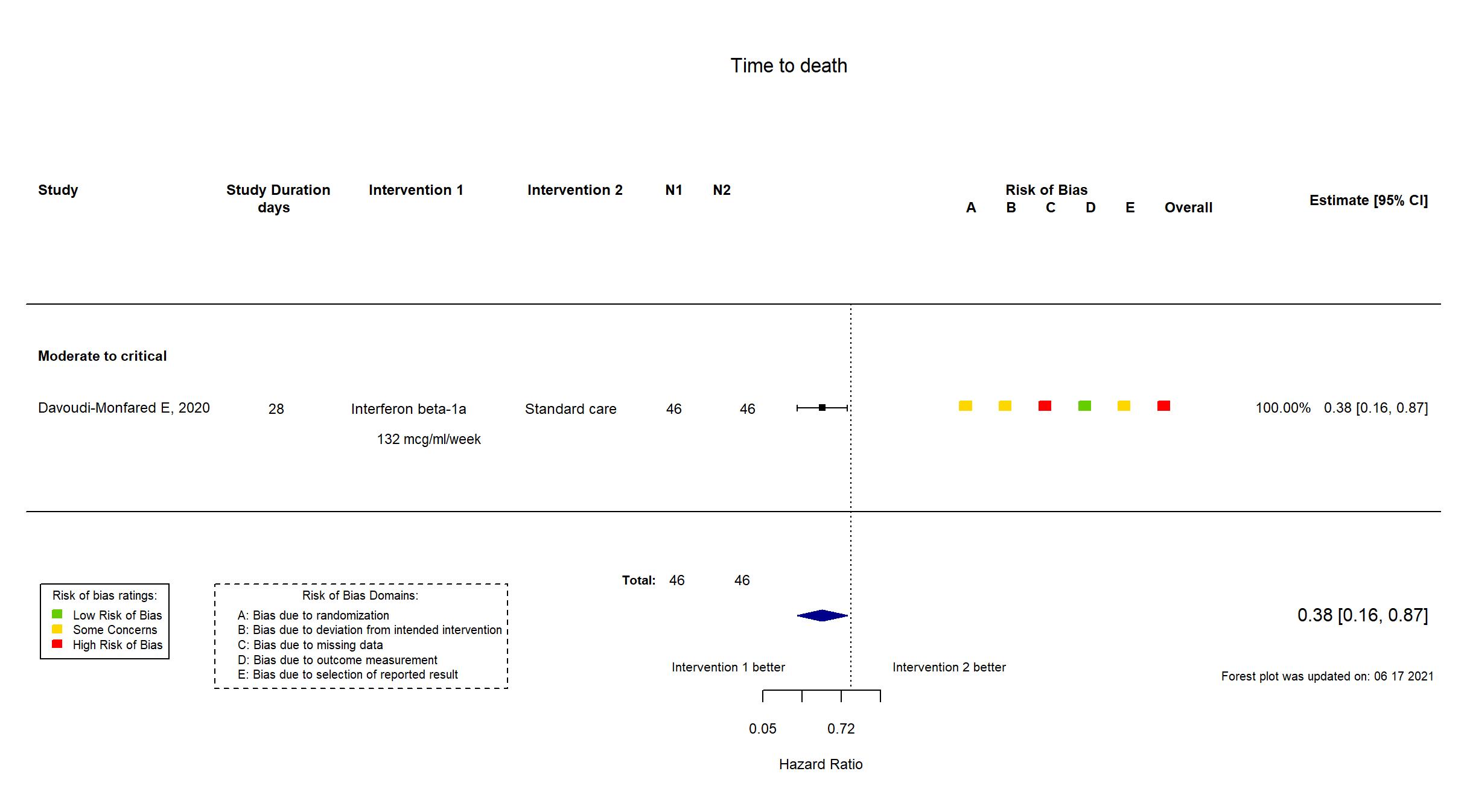




Trial NCT04343768
Publication COVIFERON - Darazam IA, Scientific Reports (2021) (published paper)
Dates: 2020-04-09 to 2020-04-30
Funding: No specific funding
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / Iran Follow-up duration (days): 21 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
INF-beta-1a+INF-beta-1b IFN beta 1b (Ziferon)(Subcutaneous injections of 0·25mg (8,000,000 IU) on days 1, 3, 6) Interferon beta-1a IFN beta 1a (Recigen) (Subcutaneous injections of 44mcg (12,000 IU) on days 1, 3, 6) Interferon beta-1b IFN beta 1b (Ziferon)(Subcutaneous injections of 0·25mg (8,000,000 IU) on days 1, 3, 6) |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : INF-beta-1a+INF-beta-1b=40 Standard care=20 Interferon beta-1a=20 Interferon beta-1b=20 | |
| Characteristics of participants N= 100 Mean age : NR 40 males Severity : Mild: n=0 / Moderate: n=* / Severe: n=* Critical: n=0 | |
| Primary outcome | |
| In the register Time to clinical improvement [ Time Frame: From date of randomization until 14 days later. ] Improvement of two points on a seven-category ordinal scale (recommended by the World Health Organization: Coronavirus disease (COVID-2019) R&D. Geneva: World Health Organization) or discharge from the hospital, whichever came first. | |
| In the report Our primary outcome measure was TTCI [time to clinical improvement], defined as the time from enrollment to discharge from the hospital or a decline of two steps on the seven-step ordinal scale. (I) Not hospitalized, and has no activity limitations; (II) Not hospitalized, but has activity limitations; (III) Hospitalized, but does not need any supplemental oxygen; (IV) Hospitalized, and needs supplemental oxygen; (V) Hospitalized, and needs either High-Flow Nasal Cannula (HFNC) or non-invasive ventilation; (VI) Hospitalized, and needs invasive ventilation; and (VII) Dead | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the pre-print article, published protocol summary and prospective trial registry were used in data extraction and assessment of risk of bias. There were some differences in outcomes and endpoints between the pre-print article, the published protocol summary and trial registry, although overall the study was reported as planned. The study achieved its pre-stated sample size. Altough the study is reported as 'open-label' it is stated that outcomce assessors were blinded to treatment arms.
On 20th of April, 2021, this study was updated based on the published report. |
Trial IRCT20100228003449N28
Publication Davoudi-Monfared E, Antimicrob Agents Ch (2020) (published paper)
Dates: 2020-02-29 to 2020-04-03
Funding: drug donation only (No funding; CinnaGen Co. (drug donation))
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / Iran Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Interferon beta-1a 44 mcg/mL subcutaneous injection, 3 times a week for 2 consecutive weeks |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Standard care=46 Interferon beta-1a=46 | |
| Characteristics of participants N= 92 Mean age : NR 43 males Severity : Mild: n=0 / Moderate: n=57 / Severe: n=4 Critical: n=20 | |
| Primary outcome | |
| In the register Response to the treatment and Complications of the treatment | |
| In the report Time to reach clinical response | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
High |
| General comment |
In addition to all available versions of the published report, the study registry and the pre-print article were used in data extraction and risk of bias assessment. The study achieved the target sample size specified in the registry. The primary outcome indicated in registry does not reflect the primary outcome reported in the paper. There is a discrepancy between the number of events in 28-day mortality reported in Table 5 and the number of deaths on day 28 reported in Table 4. The extraction and risk of bias assessment for this study was updated with the published report on 17/07/2020. |
Trial ISRCTN83971151; NCT04315948
Publication SOLIDARITY (IFN) - Pan H, N Engl J Med (2020) (published paper)
Dates: 2020-03-22 to 2020-10-04
Funding: Mixed (World Health Organization; Merck KGaA and Faron (drug donation))
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Multinational (30) Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Interferon beta-1a 44 mcg subcutaneous injection on day of randomization, day 3 and day 6 |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Interferon beta-1a=2063 Standard care=2064 | |
| Characteristics of participants N= 4127 Mean age : NR 2581 males Severity : Mild: n=972 / Moderate: n=* / Severe: n=* Critical: n=* | |
| Primary outcome | |
| In the register All-cause mortality, subdivided by the severity of disease at the time of randomization, measured using patient records throughout the study | |
| In the report In-hospital mortality | |
| Documents avalaible |
Protocol Yes. In English Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Low |
| General comment |
In addition to all available versions of the published manuscript, the pre-print article, the study registry and protocol were used in data extraction and risk of bias assessment. This was a report of interim results of an adaptive trial evaluating 4 drugs: Remdesivir, Hydroxychloroquine, Lopinavir, and Interferon-β1a. No target sample size was pre-specified.
Quote: "The protocol stated “The larger the number entered the more accurate the results will be, but numbers entered will depend on how the epidemic develops… it may be possible to enter several thousand hospitalised patients with relatively mild disease and a few thousand with severe disease, but realistic, appropriate sample sizes could not be estimated at the start of the trial.” The Executive Group, blind to any findings, decided the timing of release of interim results." Quote: "The hydroxychloroquine, lopinavir, and interferon regimens were discontinued for futility on, respectively, June 19, July 4, and October 16, 2020." Of the 2063 patients allocated to the active interferon arm, 1412 were allocated interferon and local standard of care, while 651 patients were allocated interferon and lopinavir. Furthermore, of the 2064 patients allocated to the control arm for interferon, 1385 were allocated local standard of care, while 679 were allocated lopinavir. There was no change from the trial registration in the intervention and control treatments, nor in the primary and secondary outcomes. QUote: "The regimen for interferon (mainly subcutaneous) was three doses over a period of 6 days (the day of randomization and days 3 and 6) of 44 μg of subcutaneous interferon beta-1a; where intravenous interferon was available, patients receiving high-flow oxygen, ventilation, or extracorporeal membrane oxygenation (ECMO) were instead to be given 10 μg intravenously daily for 6 days." This study was updated on December 9th using data from the published manuscript. |
Trial IRCT20100228003449N27
Publication Rahmani H, Int Immunopharmacol (2020) (published paper)
Dates: 2020-04-20 to 2020-05-20
Funding: Public/non profit (The authors did not receive any fund for this work)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / Iran Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Interferon beta-1b 250 mcg subcutaneously every other day for 14 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Interferon beta-1b=40 Standard care=40 | |
| Characteristics of participants N= 80 Mean age : NR 39 males Severity : Mild: n=0 / Moderate: n=65 / Severe: n=1 Critical: n=0 | |
| Primary outcome | |
| In the register Response to the treatment (according the clinical, paraclinical and laboratory findings); Complications of the treatment (Interview and patient's record) | |
| In the report Time to clinical improvement | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published report, the study registry was used in data extraction. The study almost achieved the target sample size reported in the registry (30 patients in each arm). There is no change from the trial registration in the intervention and control treatments. No protocol or pre-specified statistical analysis plan were available. |