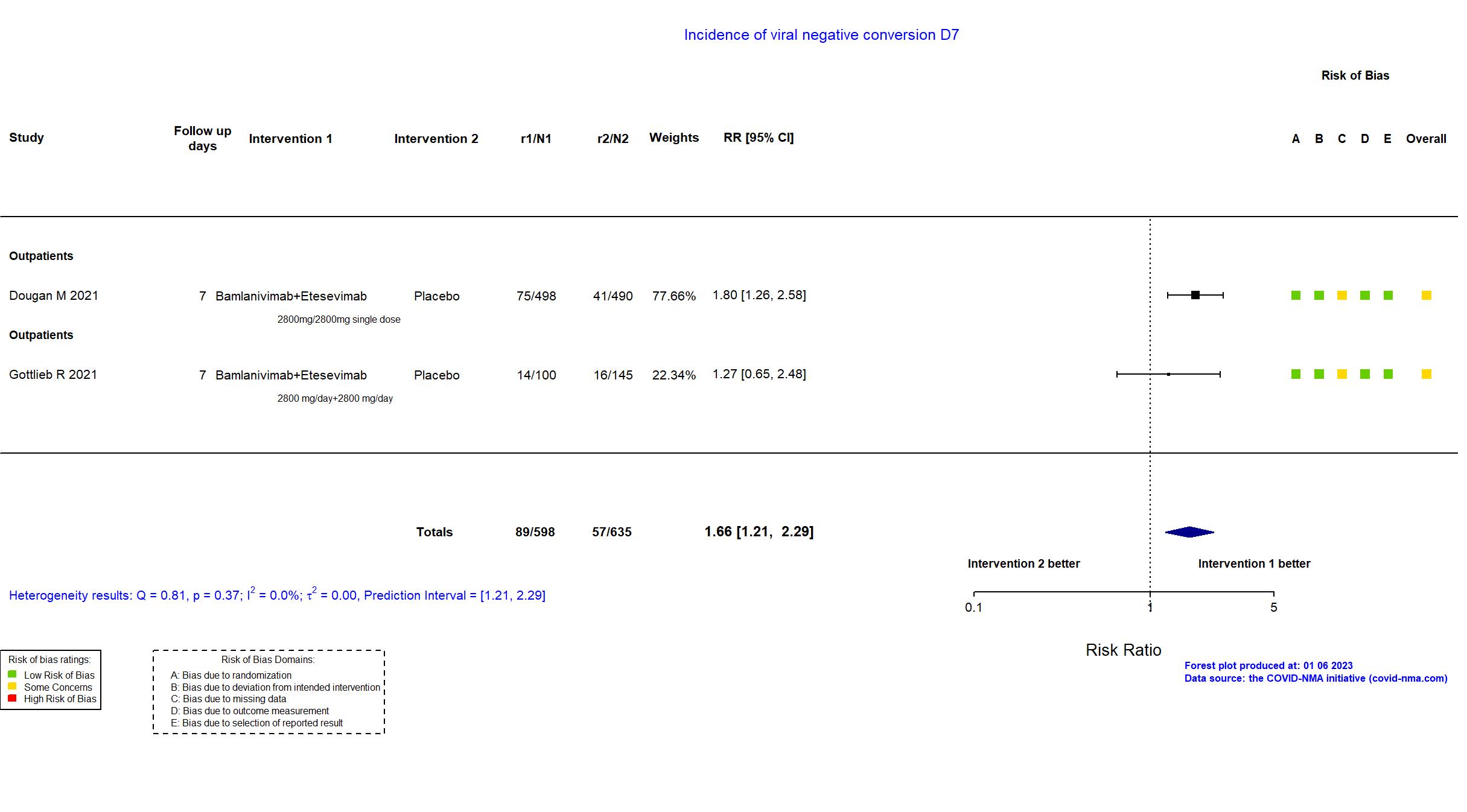
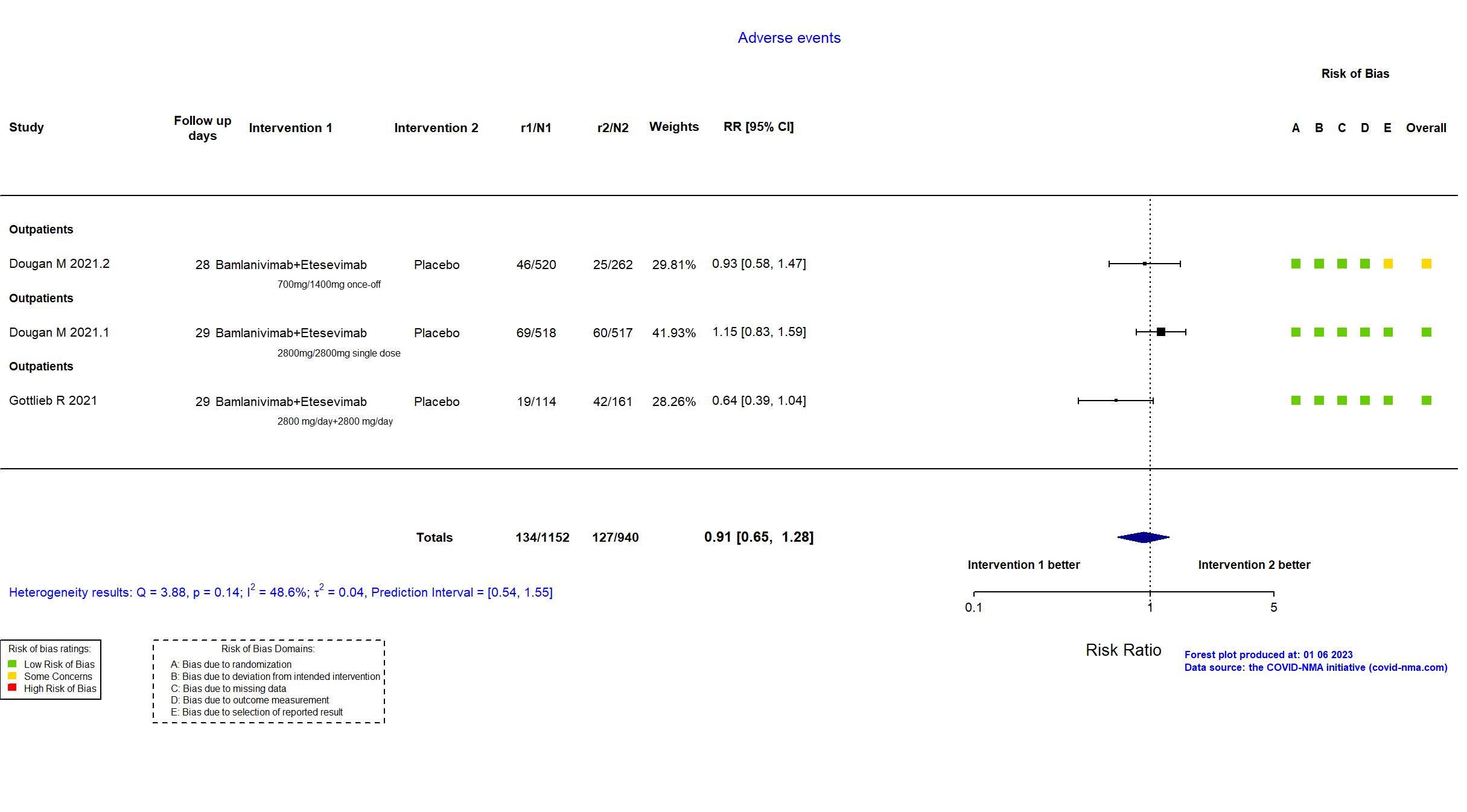
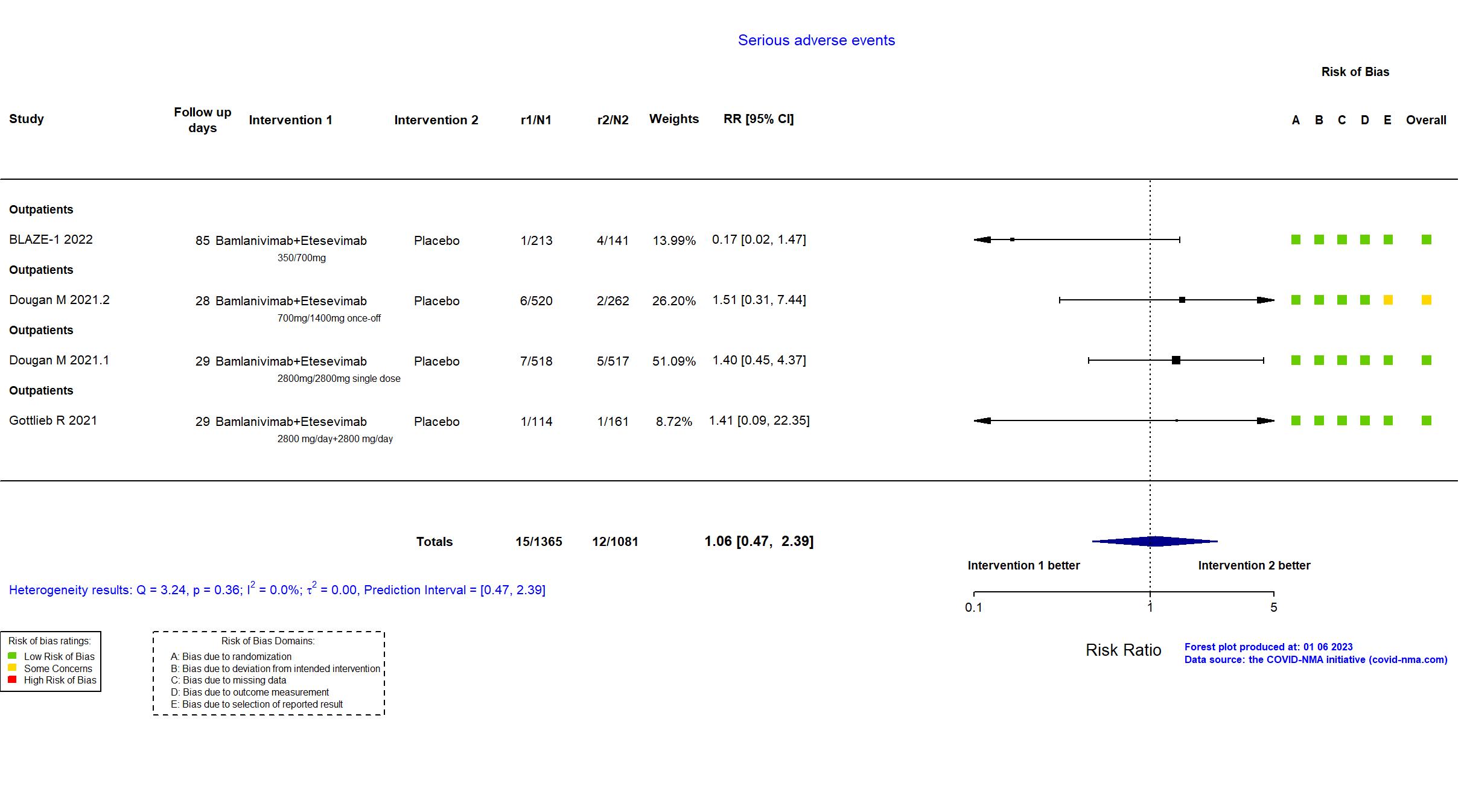
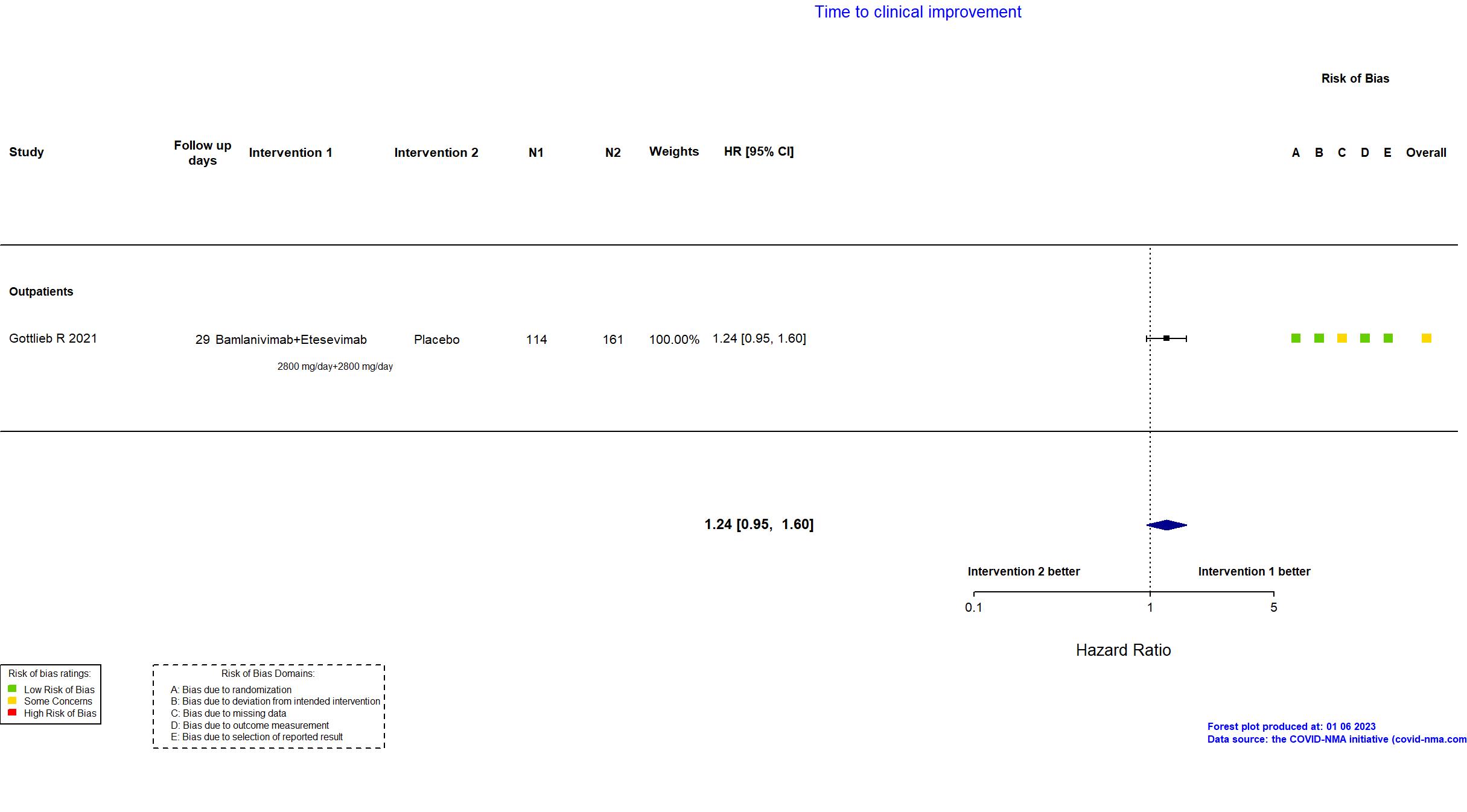
Bamlanivimab+Etesevimab vs Placebo (RCT)
Mild outpatients
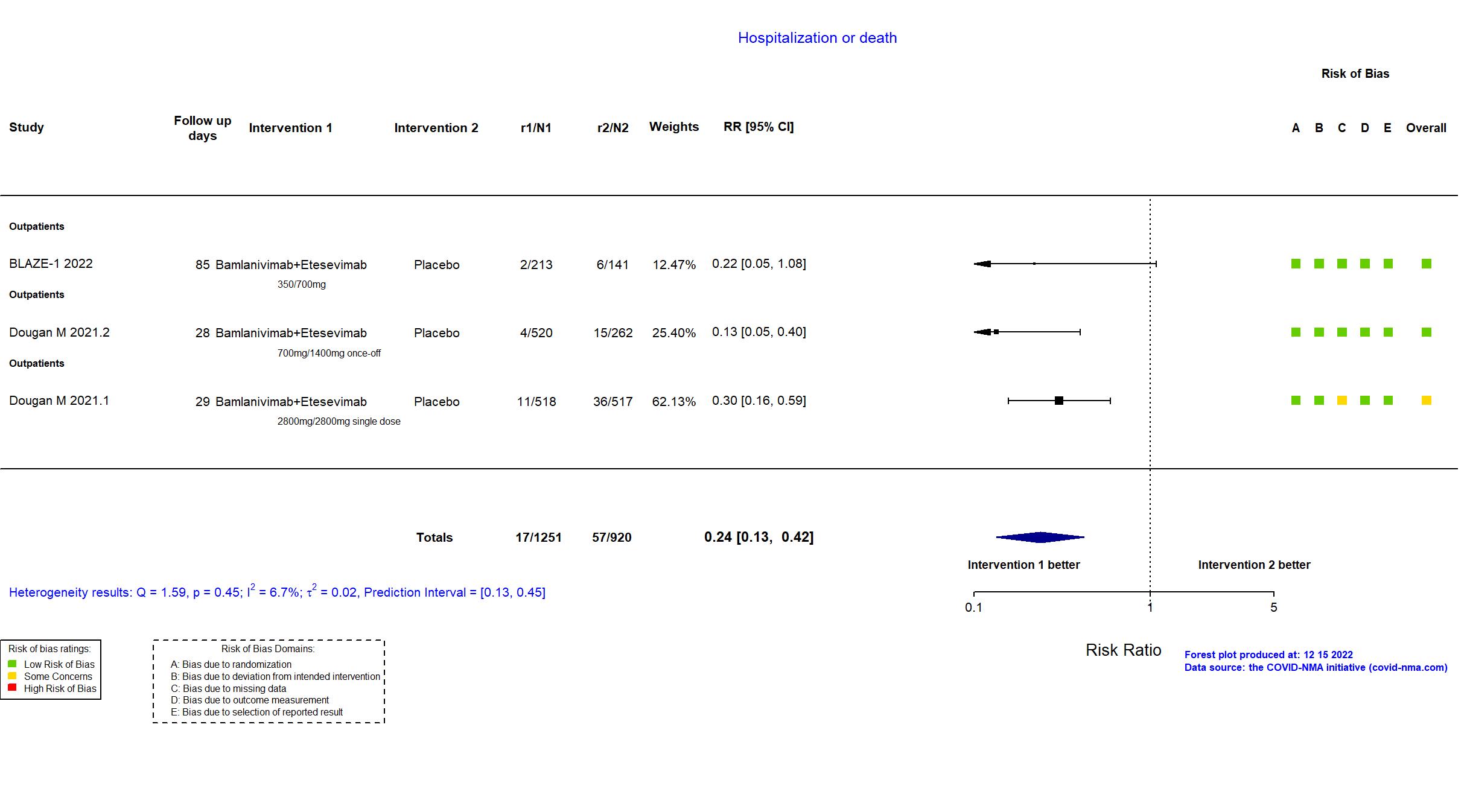
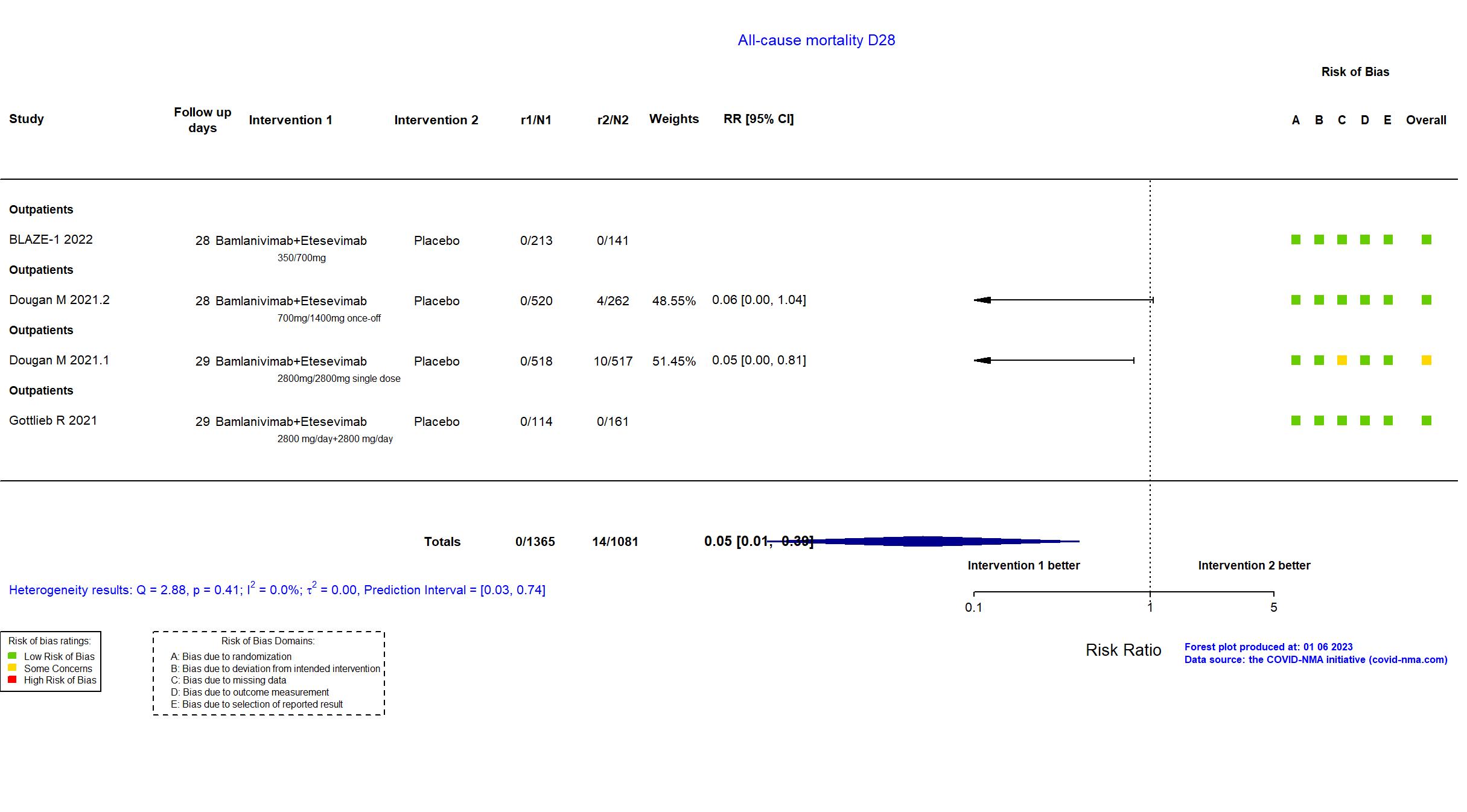
FOREST PLOTS -2023-01-06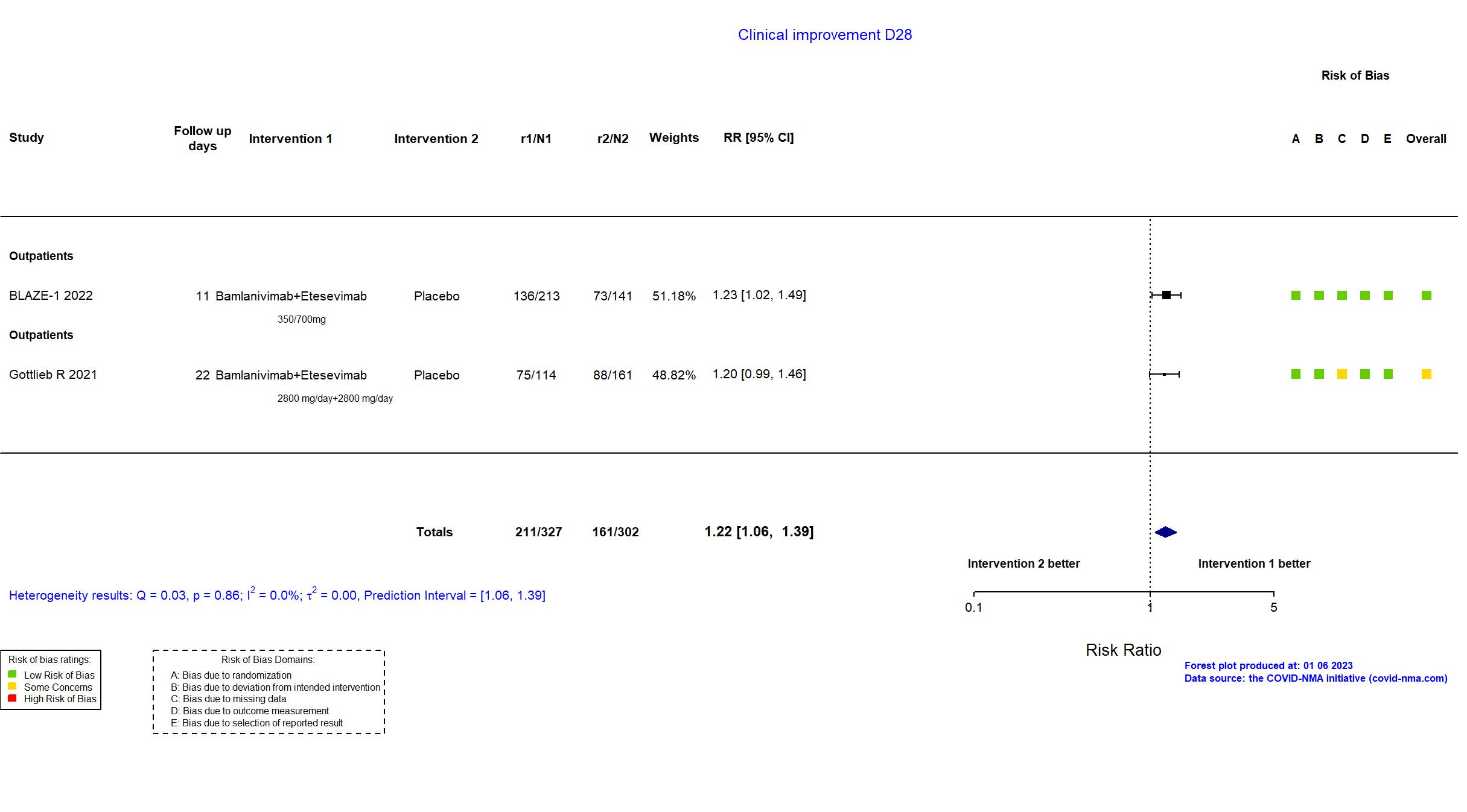










Trial NCT04427501
Publication BLAZE-1 - BLAZE-1, Unpublished (2022) (results posted on registry)
Funding: Not reported/unclear
Conflict of interest: *
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / USA Follow-up duration (days): 85 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Bamlanivimab+Etesevimab 350/700mg intravenously single dose |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Placebo=141 Bamlanivimab+Etesevimab=213 | |
| Characteristics of participants N= 354 Mean age : NR 178 males Severity : Mild: n= 354/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register Percentage of Participants With SARS-CoV-2 Viral Load Greater Than a Prespecified Threshold in Arms 350 mg Bamlanivimab/700 mg Etesevimab and Placebo [ Time Frame: Day 7 ] | |
| In the report NR | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Low |
| General comment |
This is an unpublished trial whose results have been reported in ClinicalTrials.gov. The trial registry, protocol and statistical analysis plan were used in data extraction and assessment of risk of bias.
This study reports on the phase 3 results of the Bamlanivimab 350 mg and Etesevimab 700 mg arm. Time to negative conversion and time to clinical improvement outcomes were reported but the HRs do not include the CI. |
Trial NCT04427501
Publication BLAZE-1 - Dougan M, N Engl J Med (2021) (published paper)
Dates: 2020-09-04 to 2020-12-08
Funding: Private (Eli Lilly)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / USA Follow-up duration (days): 29 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Bamlanivimab+Etesevimab 2800 mg/2800mg IV single dose |
|
| Control
Placebo | |
| Participants | |
| Randomized NR Analyzed 1035 participants Bamlanivimab+Etesevimab=518 Placebo=517 | |
| Characteristics of participants N= 1035 Mean age : NR 0 males Severity : Mild: n= 800/ Asymptomatic: n=* | |
| Primary outcome | |
| In the register Percentage of Participants Who Experience COVID-Related Hospitalization or Death from Any Cause [ Time Frame: Baseline through Day 29 ]; Change from Baseline to Day 11 in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Viral Load [ Time Frame: Baseline, Day 11 ]; Percentage of Participants with SARS-CoV-2 Viral Load Greater than a Prespecified Threshold [ Time Frame: Day 7 ]; Pharmacokinetics (PK): Area Under the Concentration-time Curve from 0 to Infinity (AUC0-inf) for both LY3819253 and LY3832479 [ Time Frame: Baseline through Day 85 ]; Percentage of Participants who Experience a Serious Adverse Event(s) SAE(s) [ Time Frame: Baseline through Day 85 ] | |
| In the report Covid-19–related hospitalization (acute care for ≥24 hours) or death from any cause by day 29 | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: N |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published article, the prospective registry, protocol and statistical analysis plan and supplementary materials were used in data extraction and assessment of risk of bias.
Some primary outcomes in the registry are reported as secondary outcomes in the report, and some secondary outcomes in the registry (proportion of patients demonstrating symptom resolution or improvement, pharmacokinetics) are not reported. Otherwise there were no substantive differences in population, procedures, interventions or outcomes between the registry, protocol and statistical analysis plan and the published article. The study achieved its target sample size.
On November 3rd, 2022, this study was updated with results published in the trial registry. |
Trial NCT04427501
Publication Dougan M, Clin Infect Dis (2021) (published paper)
Dates: 2020-12-09 to 2021-01-07
Funding: Private (Eli Lilly and Company)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / USA and Puerto Rico Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Bamlanivimab+Etesevimab 700mg/1400mg once-off intravenously |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Bamlanivimab+Etesevimab=520 Placebo=262 | |
| Characteristics of participants N= 782 Mean age : NR 361 males Severity : Mild: n= 769/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register Percentage of Participants Who Experience COVID-Related Hospitalization or Death from Any Cause [ Time Frame: Baseline through Day 29 ] Change from Baseline to Day 11 in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Viral Load [ Time Frame: Baseline, Day 11 ] Percentage of Participants with SARS-CoV-2 Viral Load Greater than a Prespecified Threshold [ Time Frame: Day 7 ] Pharmacokinetics (PK): Area Under the Concentration-time Curve from 0 to Infinity (AUC0-inf) for both LY3819253 and LY3832479 [ Time Frame: Baseline through Day 85 ] Percentage of Participants who Experience a Serious Adverse Event(s) SAE(s) [ Time Frame: Baseline through Day 85 ] | |
| In the report proportion of patients that experienced a COVID-19-related hospitalization (≥24 hours of acute care) or any-cause death by Day 29 | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published article, the protocol, analysis plan, supplementary materials and study registry were used in data extraction and risk of bias assessment. The study (n = 769) achieved its target sample size (n = 750). There is no change from the trial registration in the intervention and control treatments. The primary outcome in the article was only one of five primary outcomes in the registry. Some other primary outcomes reported in the registry are described in the article as secondary."
On April 7, 2022, another publication (Chen P, Open Forum Infect Dis, 2022) on the same trial was published. No COVID-NMA-defined outcomes were available/extracted from this publication. This study was updated on June 9, 2022 to correct an error in the risk of bias. |
Trial NCT04427501
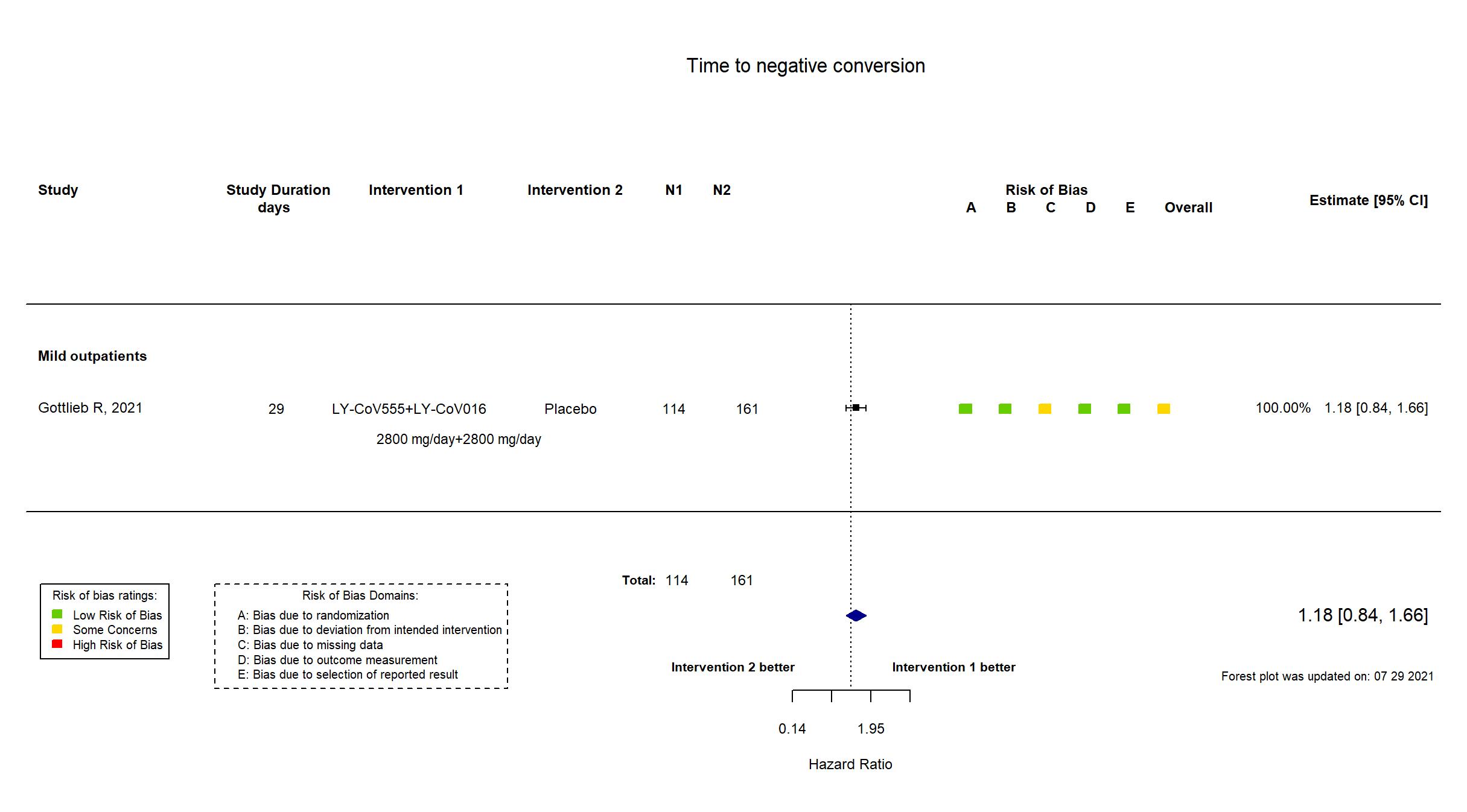
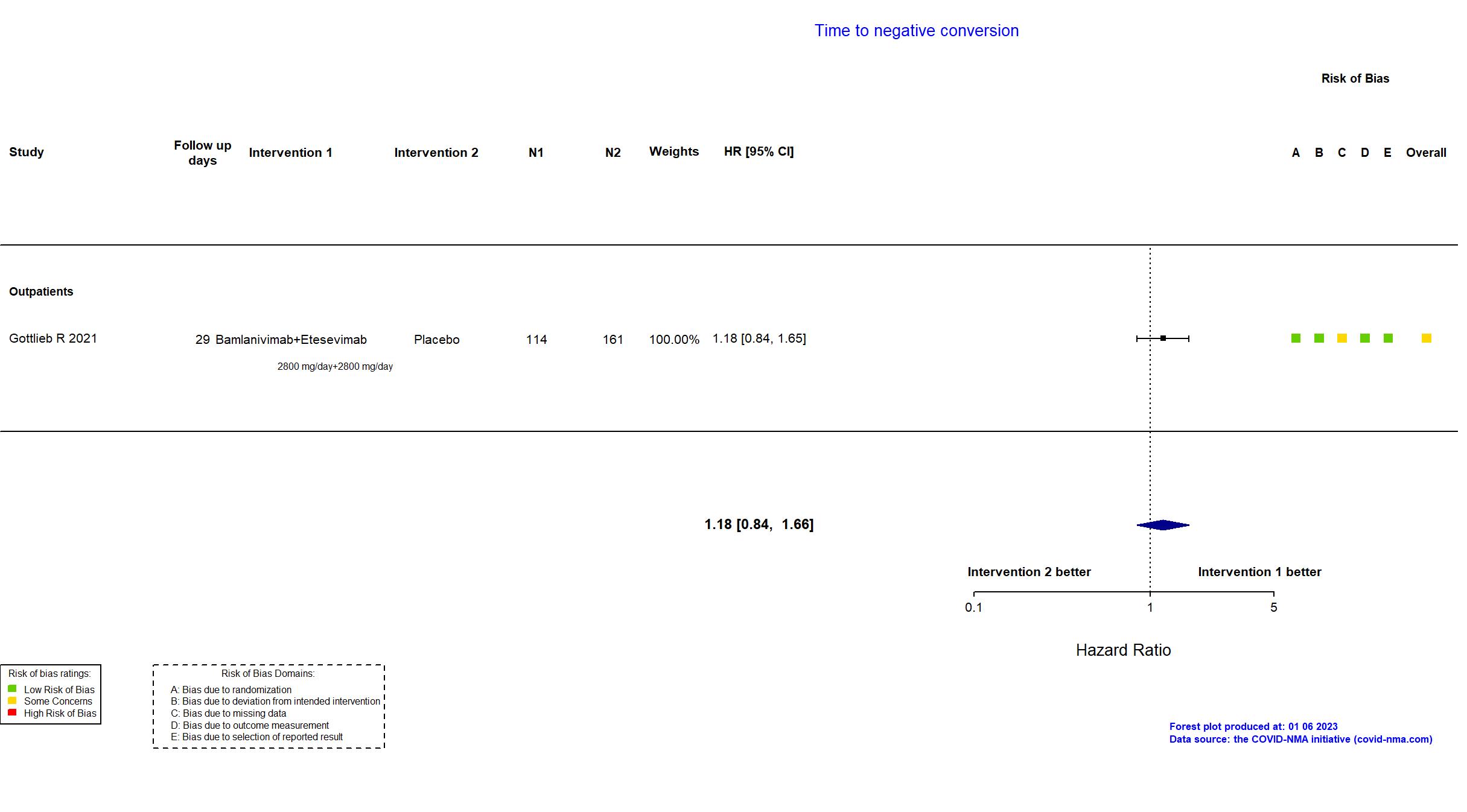
Publication BLAZE-1 - Gottlieb R, JAMA (2021) (published paper)
Dates: 2020-06-17 to 2020-09-03
Funding: Private (Eli Lilly and Company)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Participants, outcome assessor and health care pro | |
| Location :
Multicenter / USA Follow-up duration (days): 29 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Bamlanivimab 700 mg IV single dose for 1 hour Bamlanivimab 700 mg 700 mg IV single dose for 1 hour Bamlanivimab 2800 mg 2800 mg IV single dose for 1 hour Bamlanivimab 7000 mg 7000 mg IV single dose for 1 hour Bamlanivimab+Etesevimab LY-CoV555: 2800 mg IV single dose for 1 hour LY-CoV016: 2800 mg IV single dose for 1 hour |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Bamlanivimab=317 Bamlanivimab 700 mg=104 Bamlanivimab 2800 mg=109 Bamlanivimab 7000 mg=104 Bamlanivimab+Etesevimab =114 Placebo=161 | |
| Characteristics of participants N= 909 Mean age : NR 300 males Severity : Mild: n= 678/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register Percentage of Participants Who Experience COVID-Related Hospitalization or Death from Any Cause [ Time Frame: Baseline through Day 29 ]; Change from Baseline to Day 11 in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Viral Load [ Time Frame: Baseline, Day 11 ]; Percentage of Participants with SARS-CoV-2 Viral Load Greater than a Prespecified Threshold [ Time Frame: Day 7 ] | |
| In the report Change in SARS- CoV-2 log viral load from baseline to day 11 (+/-4 days). | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: No |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published article, the trial registry, study protocol, statistical analysis plan, and supplementary materials were used in data extraction and assessment of risk of bias. Two outcomes described as primary in the trial registry are described as secondary in the protocol and reported as secondary outcomes. Otherwise there were no substantive differences between the published article and the trial registry, study protocol ad statistical analysis plan. The database lock occurred when the last patient randomized to treatment of bamlanivimab and etesevimab reached day 29. As a result, the target sample size specified in the registry was not achieved.
This is a phase 2/3 trial of previously published trial, Chen P, N Engl J Med, 2020 included in this review. Quote: "Interim results from the Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) trial with data for the 3 monotherapy doses of the neutralizing antibody bamlanivimab have been published. The current report presents the final data set for patients randomized to the 4 treatment groups and the placebo group in the initial portion of the trial, including findings for the bamlanivimab and etesevimab combination group, the 3 bamlanivimab monotherapy groups, and the placebo group." This study was updated on November 3rd, 2022 with the results published in the trial registry. |