Remdesivir 5 days vs Remdesivir 10 days (RCT)
Hospitalized patients
FOREST PLOTS -2022-10-11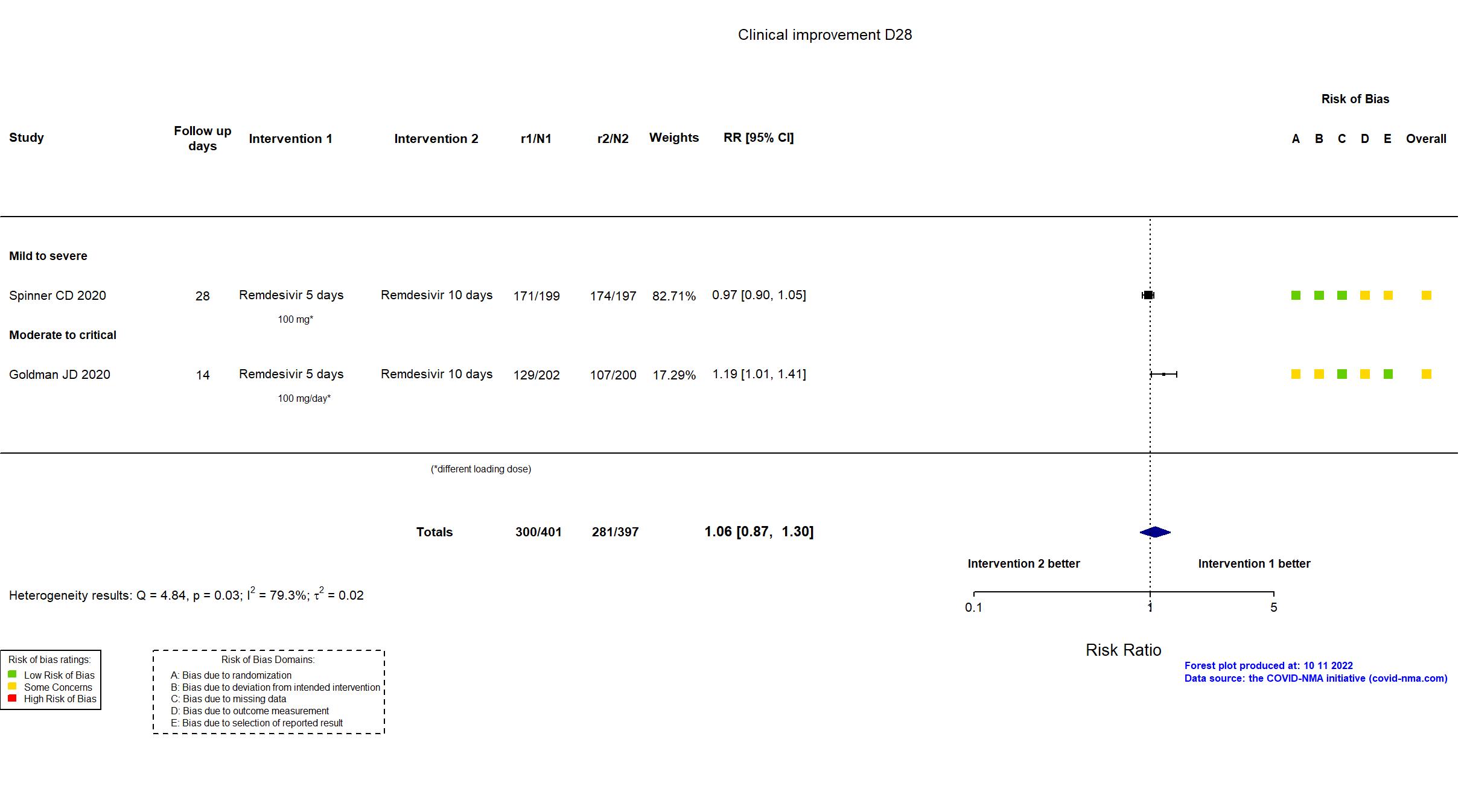
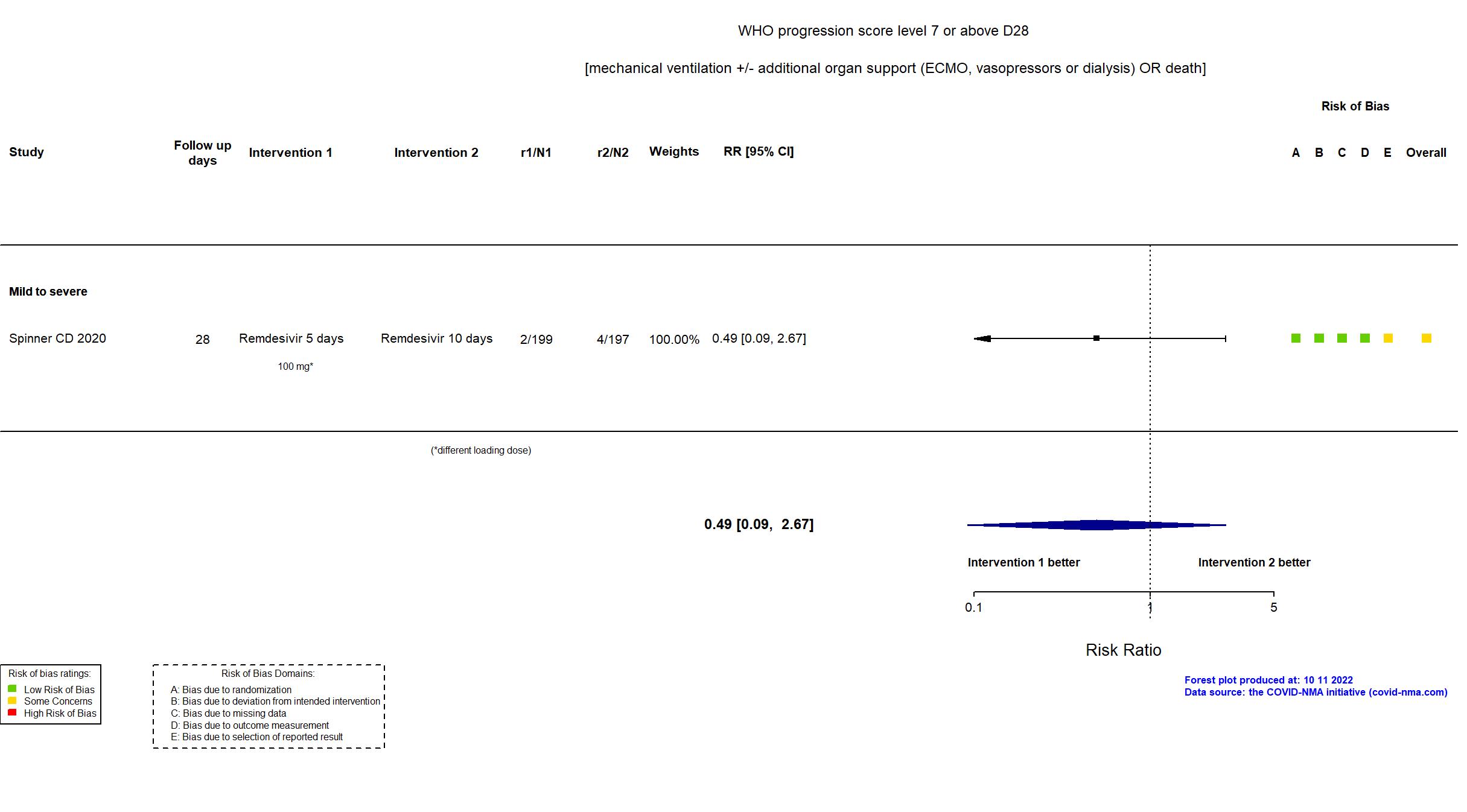
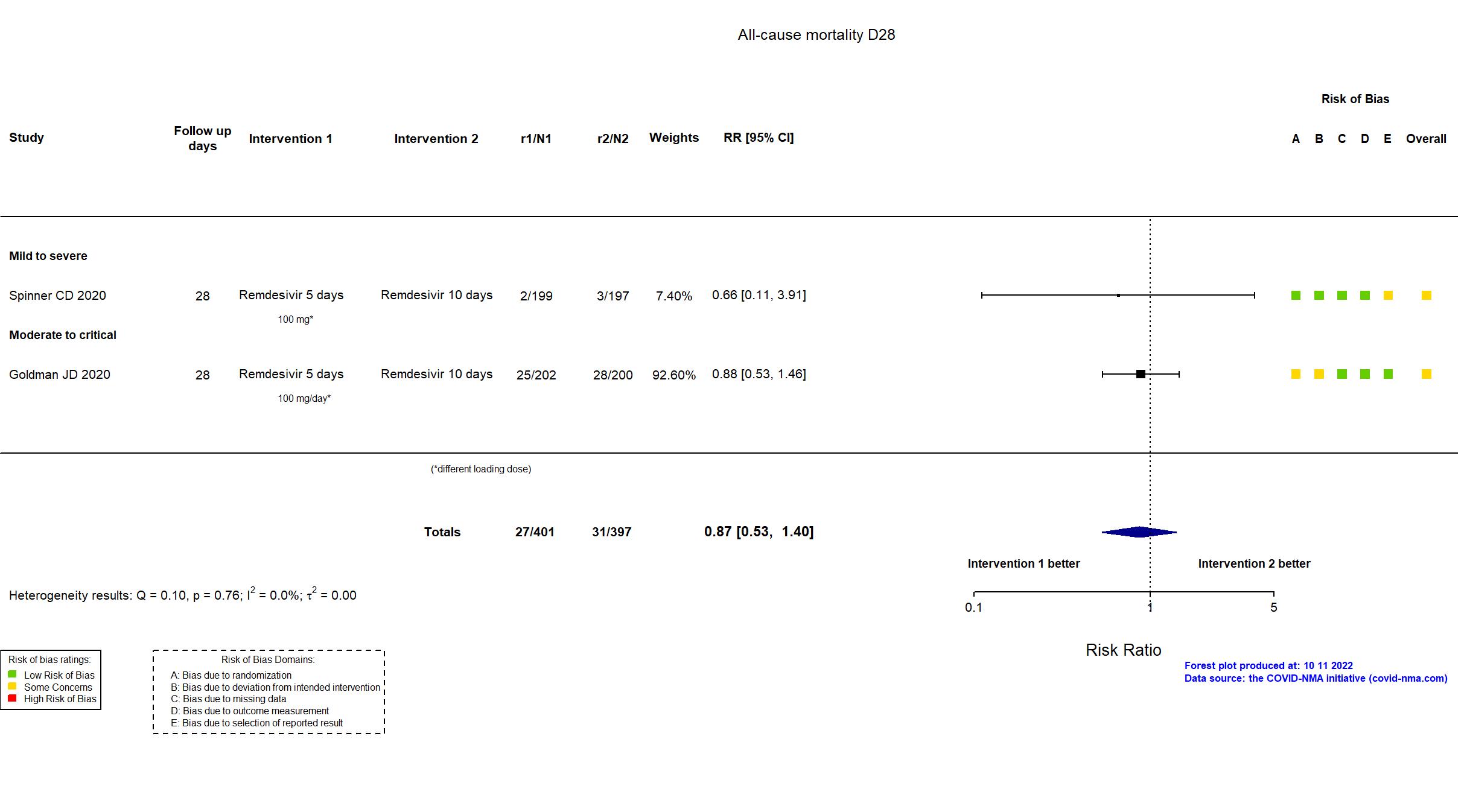

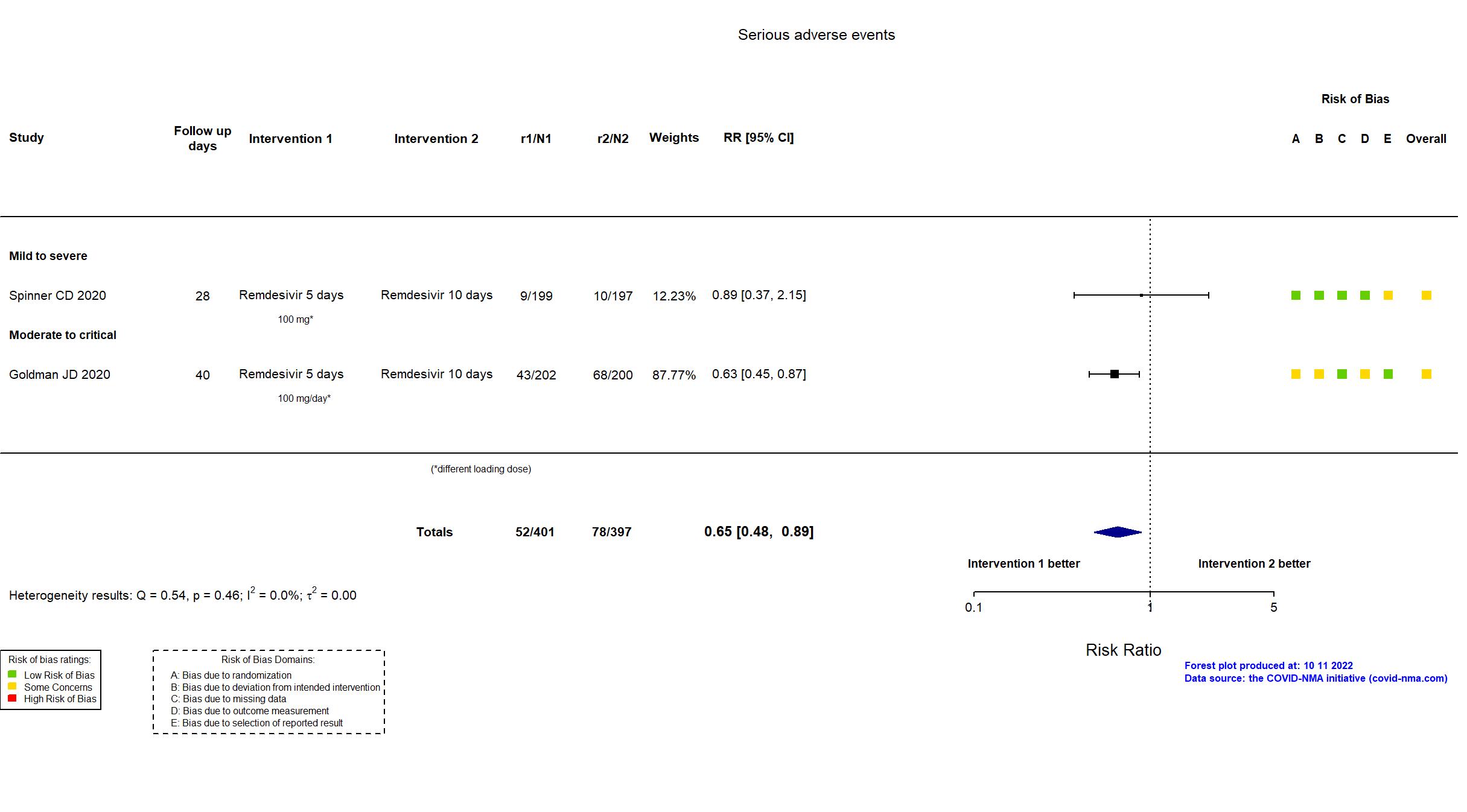








Trial NCT04292899
Publication Goldman JD, N Engl J Med (2020) (published paper)
Dates: 06mar2020 to 26mar2020
Funding: Private (Gilead Sciences)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / United States, Italy, Spain, Germany, Hong Kong, Singapore, South Korea, and Taiwan Follow-up duration (days): 40 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Remdesivir 5 days 200 mg IV on day 1 followed by 100 mg once a day for the next 4 days. |
|
| Control
Remdesivir 10 days 200 mg IV on day 1 followed by 100 mg once a day for the next 9 days. | |
| Participants | |
| Randomized participants : Remdesivir 10 days=200 Remdesivir 5 days=202 | |
| Characteristics of participants N= 402 Mean age : NR 253 males Severity : Mild: n=0 / Moderate: n=275 / Severe: n=109 Critical: n=13 | |
| Primary outcome | |
| In the register The odds ratio for improvement on a 7 point ordinal scale on day 14 | |
| In the report Clinical status assessed on day 14 on a 7-point ordinal scale consisting of the following categories: 1, death; 2, hospitalized, receiving invasive mechanical ventilation or ECMO; 3, hospitalized, receiving noninvasive ventilation or high-flow oxygen | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to all available versions of the published/pre-print article, the study registry, protocol, and statistical analysis plan were used in data extraction and risk of bias assessment. The primary outcome in the report reflects the primary outcome indicated in the registry. The trial registration describes additional trial arms as an extension to the study (the additional arms would enroll participants after enrollment to Part A, that includes the two arms presented in the published report, was complete). The extraction was updated on January 20th, 2021 based on results published on Clinicaltrials.gov on December 31st, 2020. |
Trial NCT04292730
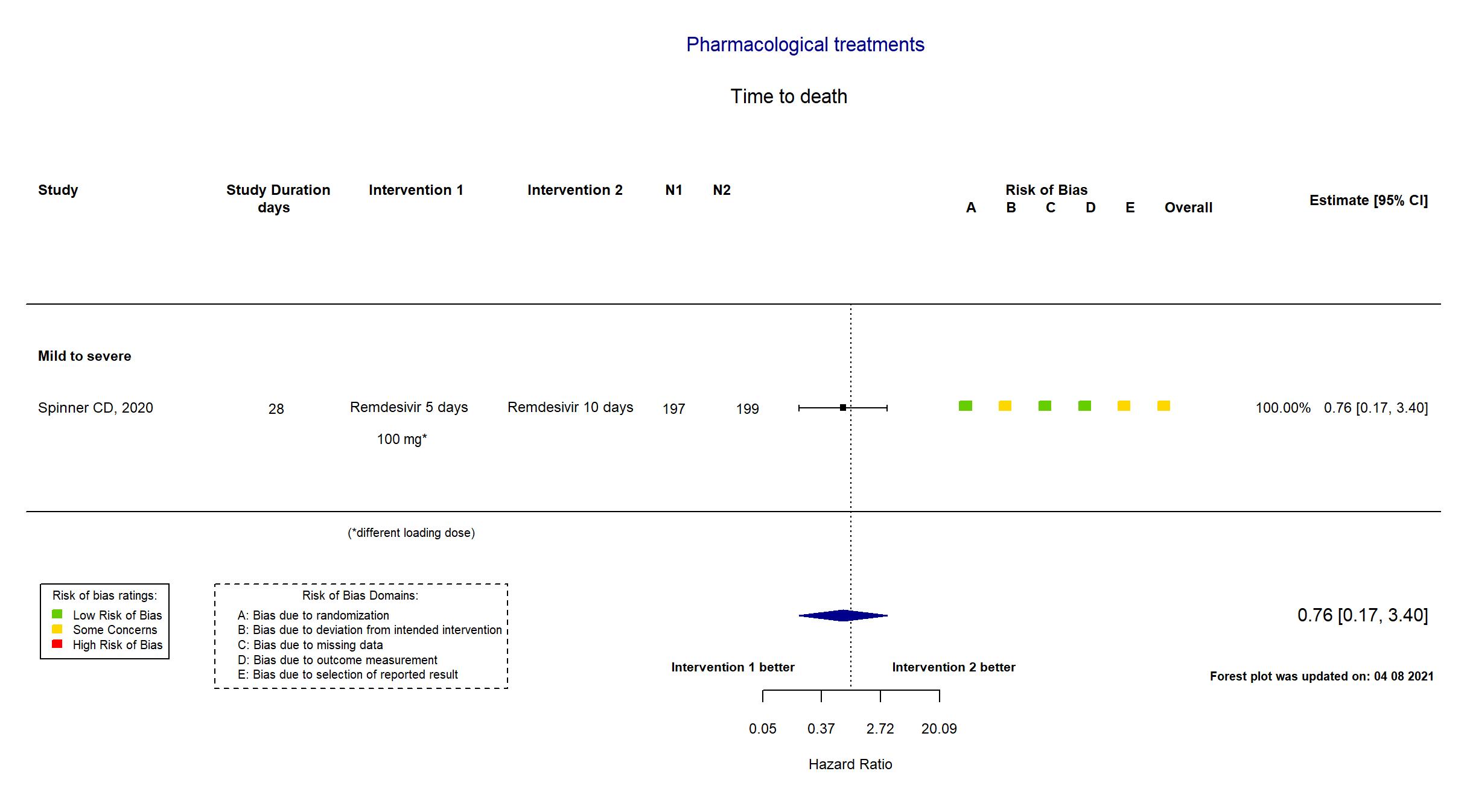
Publication Spinner CD, JAMA (2020) (published paper)
Dates: 2020-03-15 to 2020-04-18
Funding: Private (Gilead Sciences)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Multinational Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Remdesivir 200 mg IV infusion on day-1 followed by 100 mg once a day for next 9 days Remdesivir 10 days 200 mg IV infusion on day-1 followed by 100 mg once a day for next 9 days Remdesivir 5 days 200 mg IV infusion on day-1 followed by 100 mg once a day for next 4 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Remdesivir=396 Remdesivir 10 days=197 Remdesivir 5 days=199 Standard care=200 | |
| Characteristics of participants N= 992 Mean age : NR 475 males Severity : Mild: n=660 / Moderate: n=111 / Severe: n=6 Critical: n=0 | |
| Primary outcome | |
| In the register The Odds of Ratio for Improvement on a 7-point Ordinal Scale on Day 11 [ Time Frame: Day 11 ] The odds ratio represents the odds of improvement in the ordinal scale between the treatment groups. The ordinal scale is an assessment of the clinical stat | |
| In the report Clinical status assessed by a 7-point ordinal scale on Day 11 | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the study registry, protocol, SAP and supplementary files were used in data extraction and risk of bias assessment. This is a report on a multinational 3 arm RCT in Phase A and Phase B is ongoing. Quote: "In addition, a non-randomized extension phase was added in which up to 1000 additional patients could be enrolled to receive remdesivir; the results of the extension phase will be the subject of a subsequent report". The target sample size specified in the registry 1113 was not yet achieved. There is no change from the trial registration in the intervention and control treatments. Secondary outcomes in the report are not specified in the trial registry however they are congruent with the protocol. Quote: "The protocol was amended on March 15, 2020, on the basis of emerging understanding of the clinical presentation and assessment of COVID-19. The age limit for eligibility was lowered from 18 years to 12 years and the minimum temperature requirement was eliminated. The primary end point in the original protocol was the proportion of patients discharged by day 14 of the study. This was amended to assessment of clinical status on a 7-point ordinal scale by day 11 |