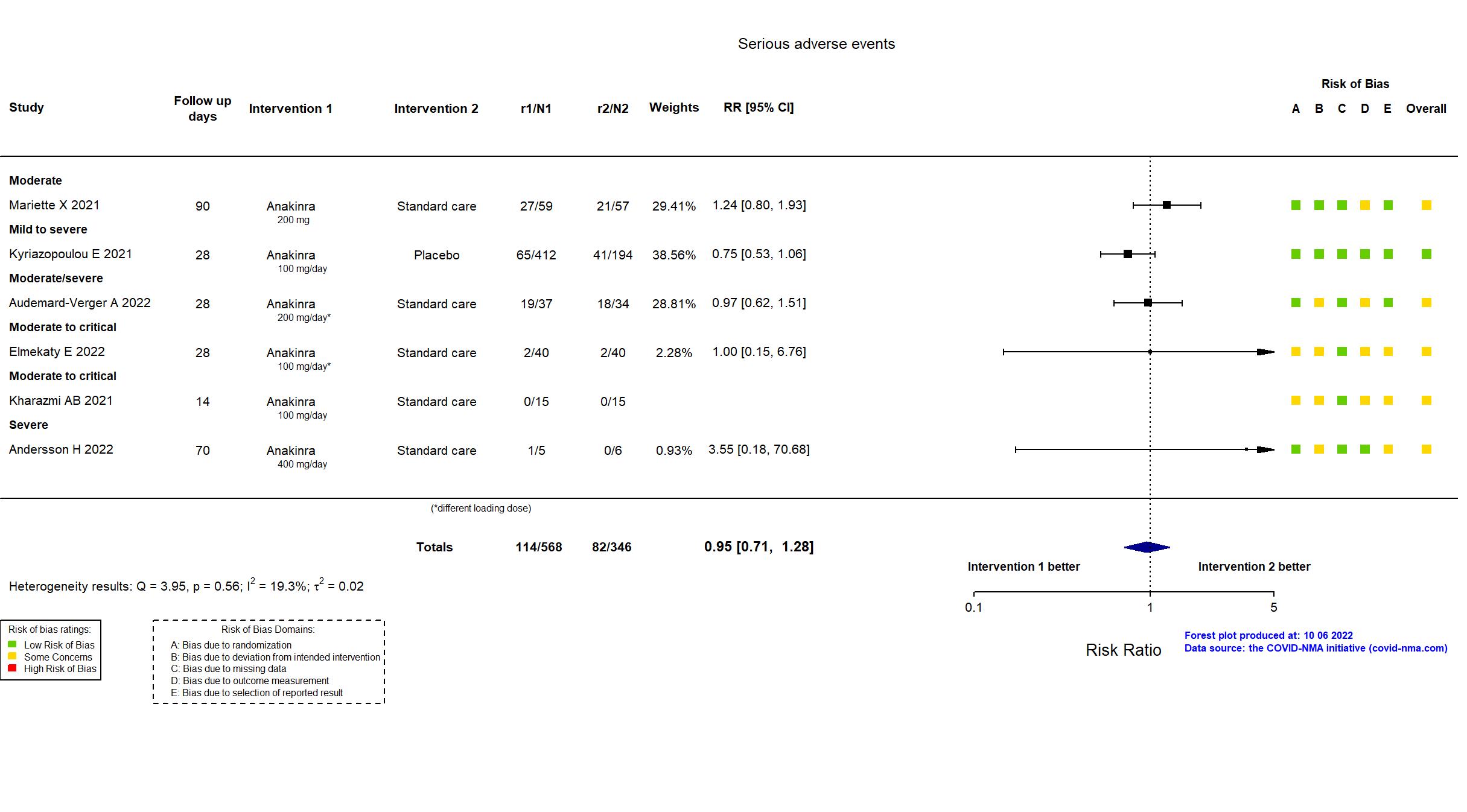
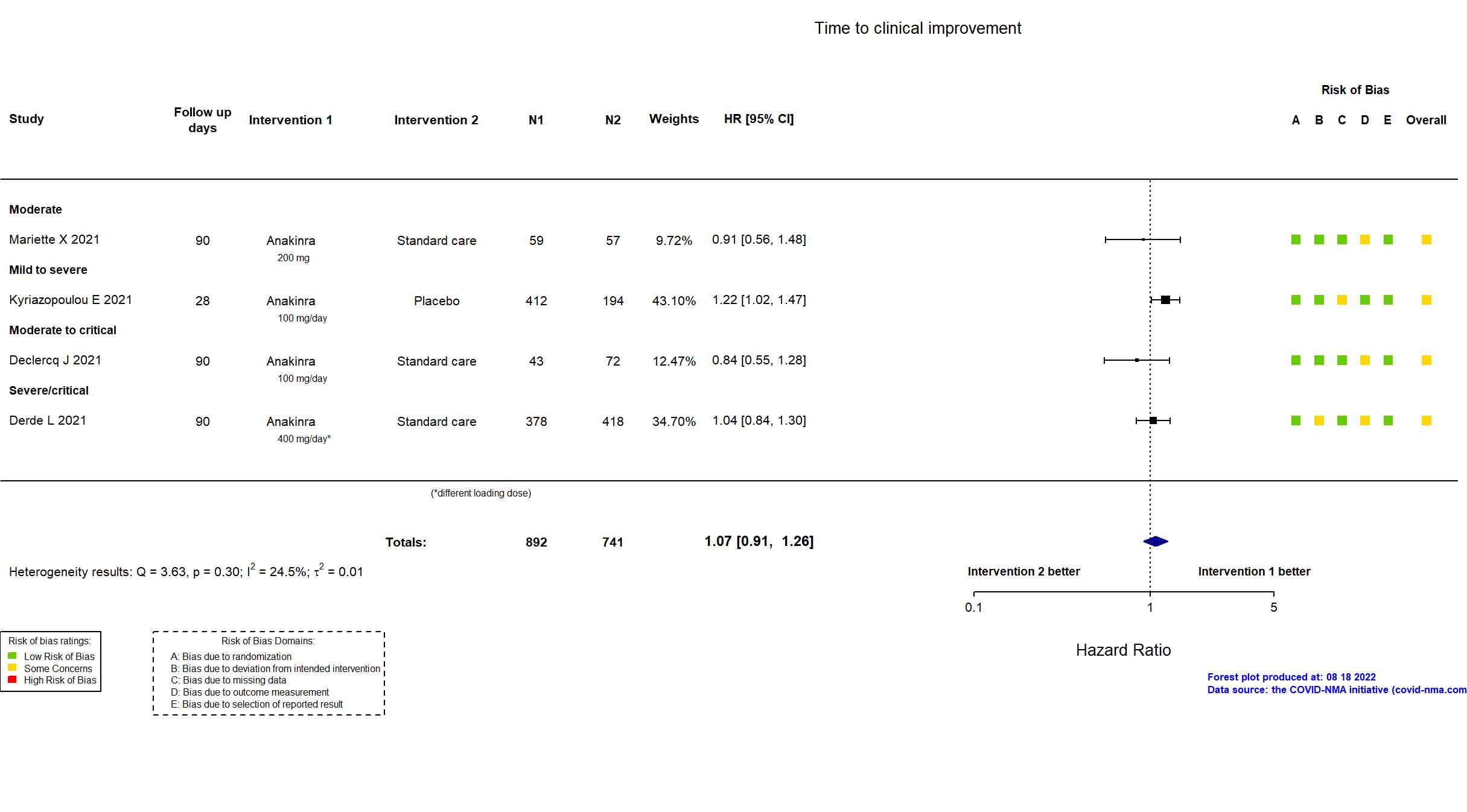
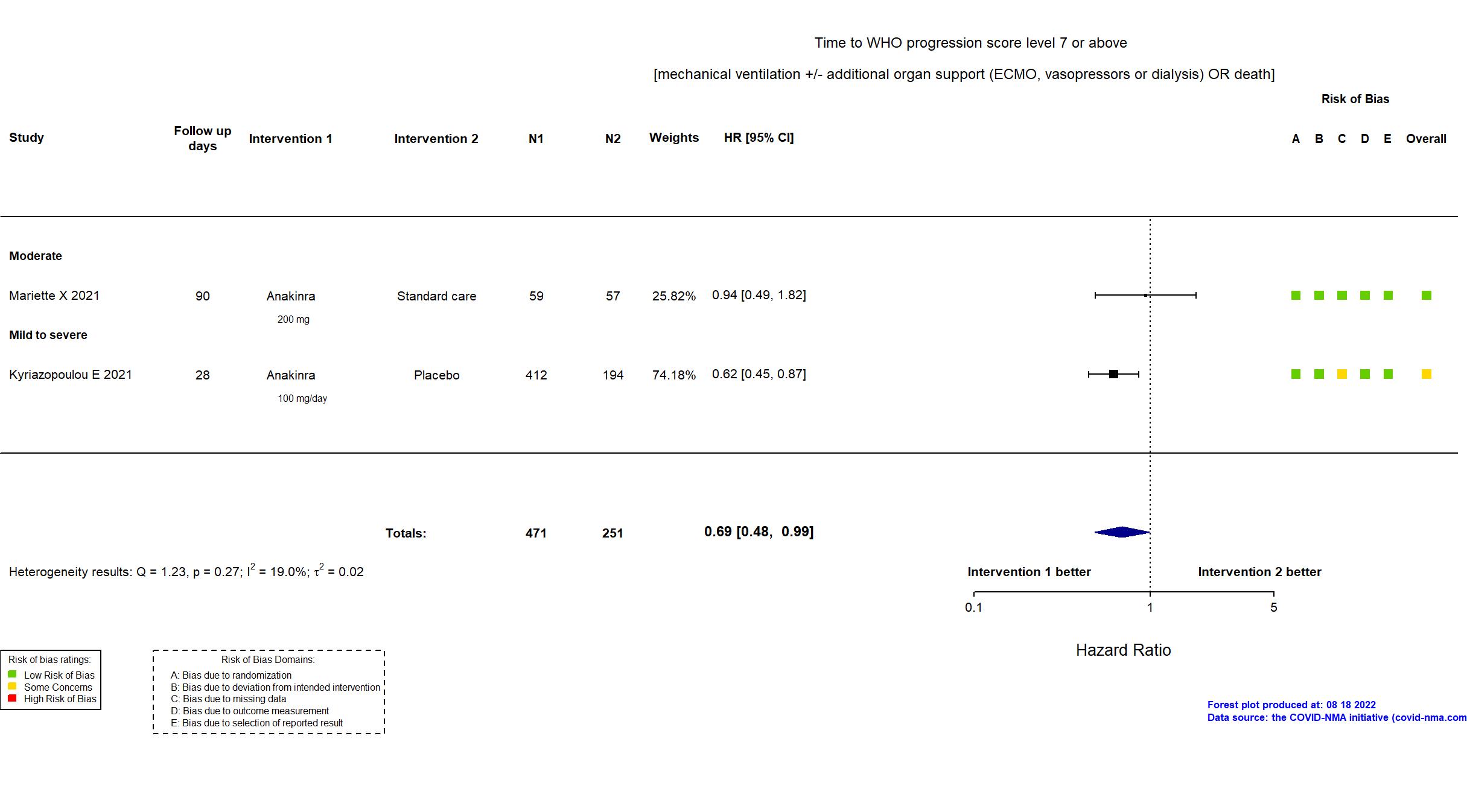
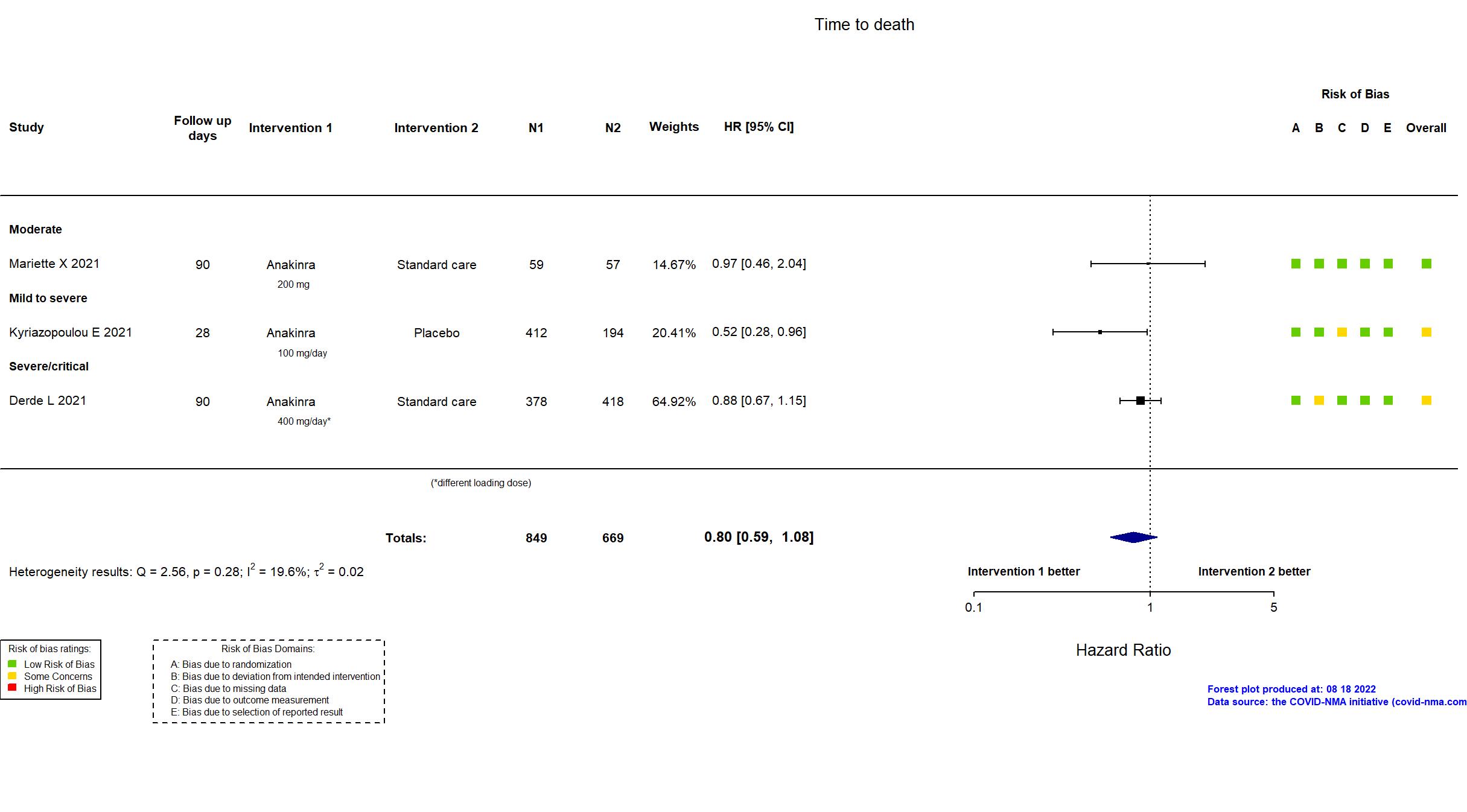
Studies description
Trial NCT04324021
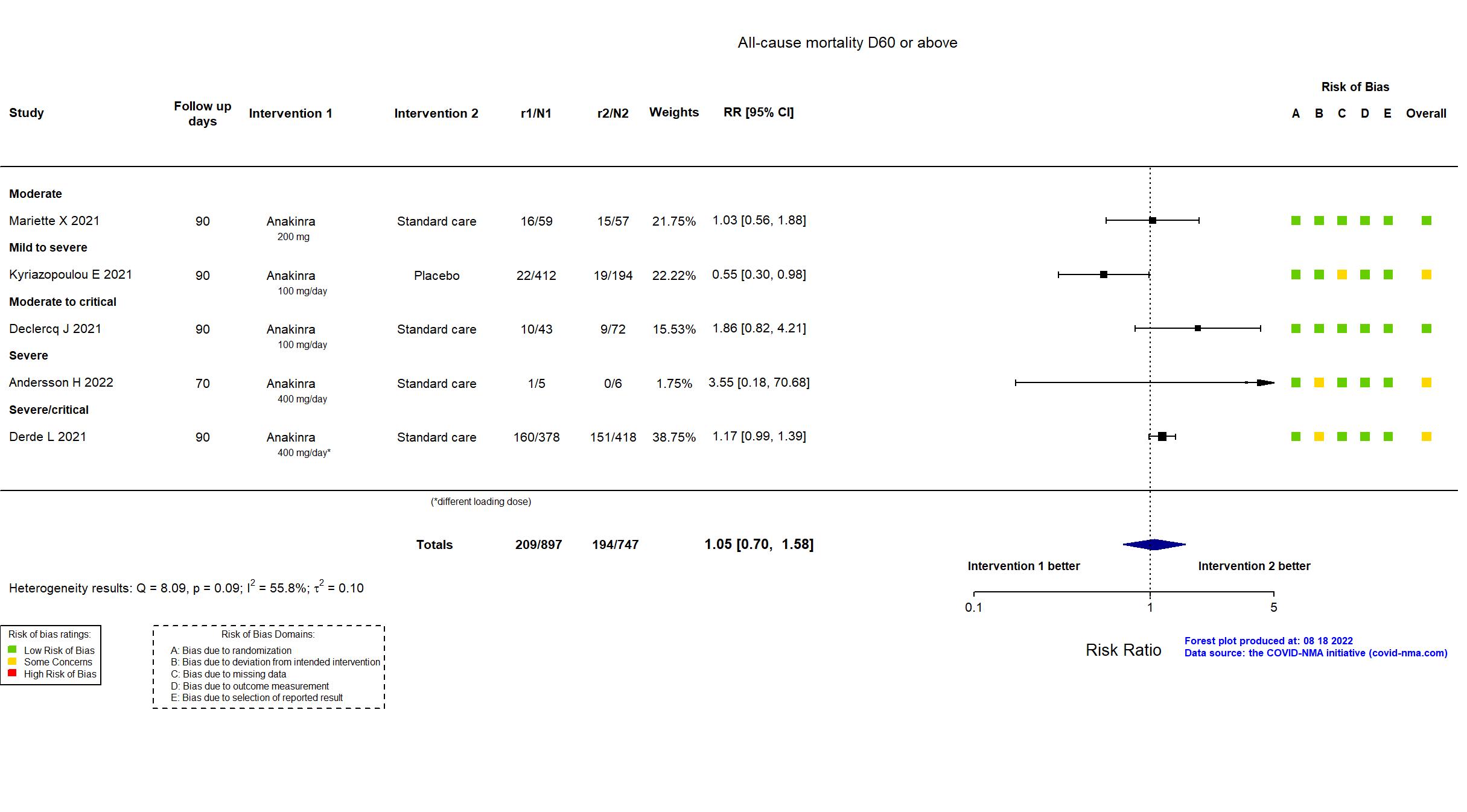
Publication Andersson H, Unpublished (2022) (results posted on registry)
Funding: Private (Swedish Orphan Biovitrum)
Conflict of interest: *
Trial NCT04364009
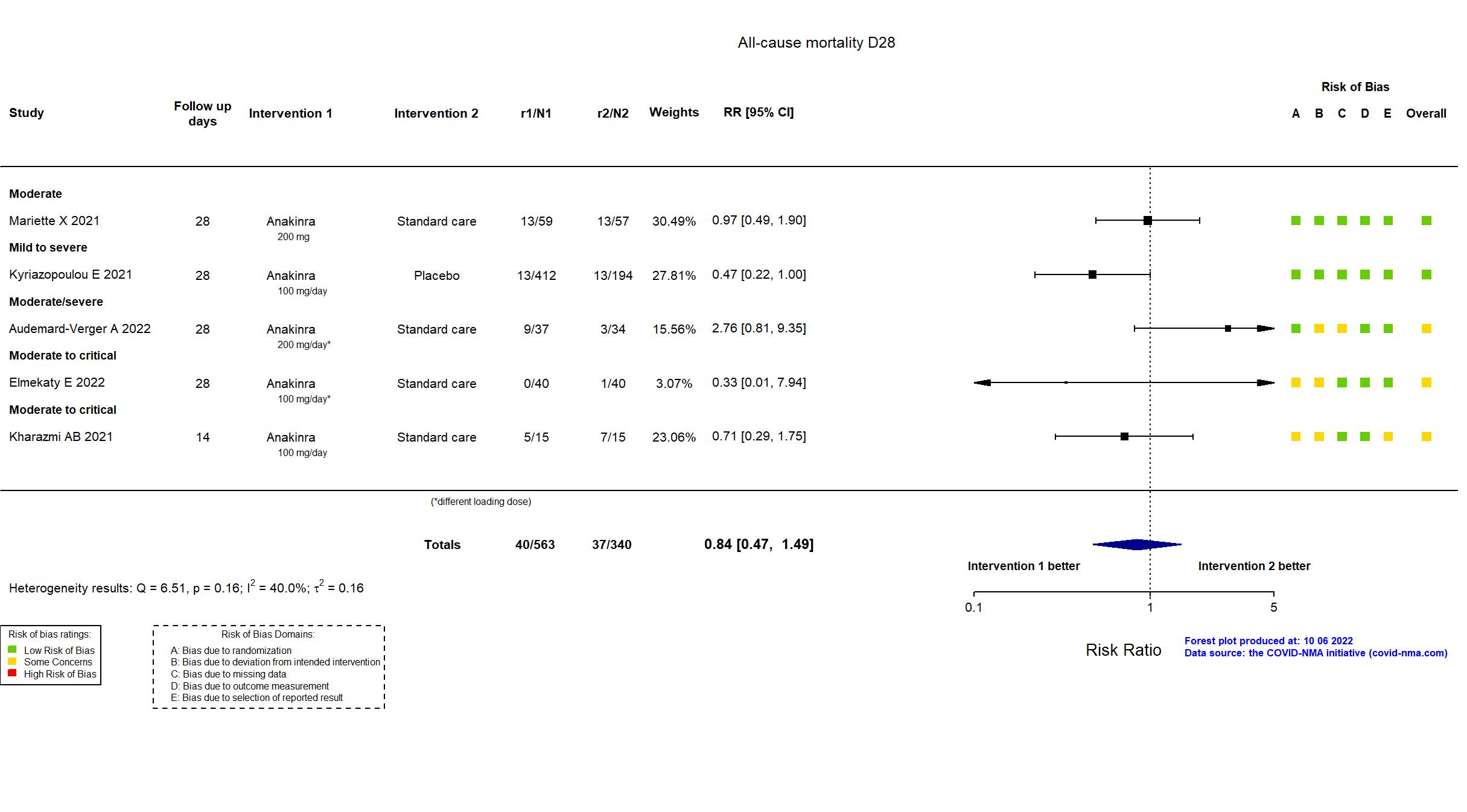
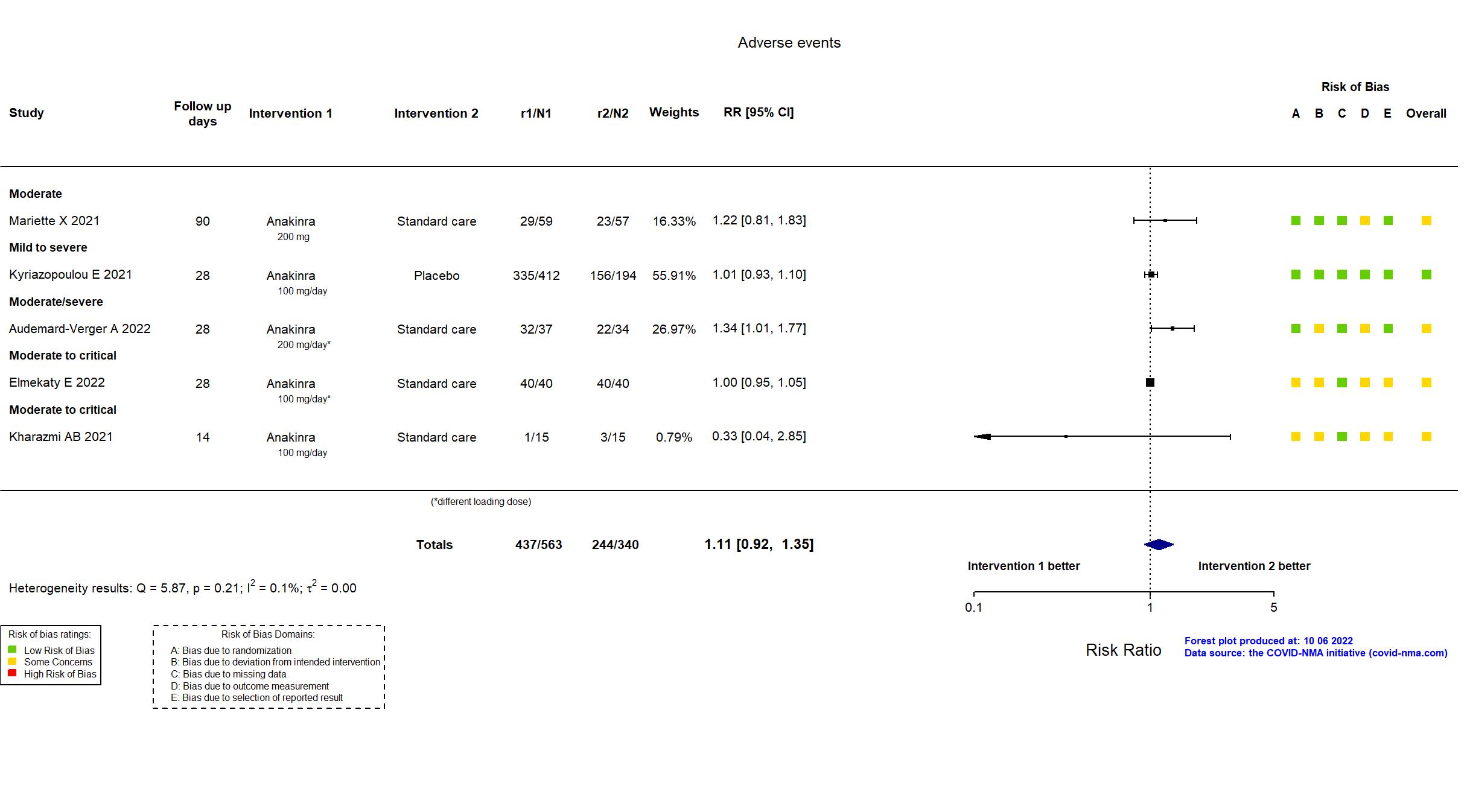
Publication ANACONDA - Audemard-Verger A, Plos One (2022) (published paper)
Dates: 2020-04-27 to 2020-10-06
Funding: Public/non profit (Tours university hospital)
Conflict of interest: No
Trial NCT04330638; EudraCT2020-001500-41
Publication COV-AID - Declercq J, Lancet Respir Med (2021) (published paper)
Dates: 2020-04-04 to 2020-12-06
Funding: Public/non profit (Belgian Health Care Knowledge Center; VIB Grand Challenges (Flemish Institute for Biotechnology))
Conflict of interest: No
Trial NCT02735707
Publication REMAP-CAP - Derde L, medRxiv (2021) (preprint)
Dates: 2020-03-25 to 2021-04-10
Funding: Mixed (PREPARE consortium by the European Union; FP7-HEALTH-2013-INNOVATION-1; RECOVER consortium by the European Union Horizon 2020 research and innovation program; Australian National Health and Medical Research Council; Health Research Council of New Zealand; Canadian Institute of Health Research Strategy for Patient-Oriented Research Innovative Clinical Trials Program Grant; UK NIHR; NIHR Imperial Biomedical Research Centre; Health Research Board of Ireland; UPMC Learning While Doing Program; Translational Breast Cancer Research Consortium; Global Coalition for Adaptive Research; French Ministry of Health; Minderoo Foundation; Wellcome Trust Innovations Project; Netherlands Organization for Health Research and Development ZonMw; NIHR Research Professorship; NIHR Clinician Scientist Fellowship; Australian National Health and Medical Research Council Career Development Fellowship; Roche Products Ltd; Sanofi (Aventis Pharma Ltd); Swedish Orphan Biovitrum AB (Sobi); Faron Pharmaceuticals (drug provision in some countries)
)
Conflict of interest: No
Trial NCT04643678
Publication Elmekaty E, medRxiv (2022) (preprint)
Dates: 2020-10-30 to 2021-04-30
Funding: Public/non profit (Hamad Medical Corporation, Qatar)
Conflict of interest: No
Trial IRCT20120703010178N20
Publication Kharazmi AB, Immun Inflamm Dis (2021) (published paper)
Dates: 2020-05-01 to 2020-07-30
Funding: Private (Persisgen Par Pharmaceutical Company)
Conflict of interest: No
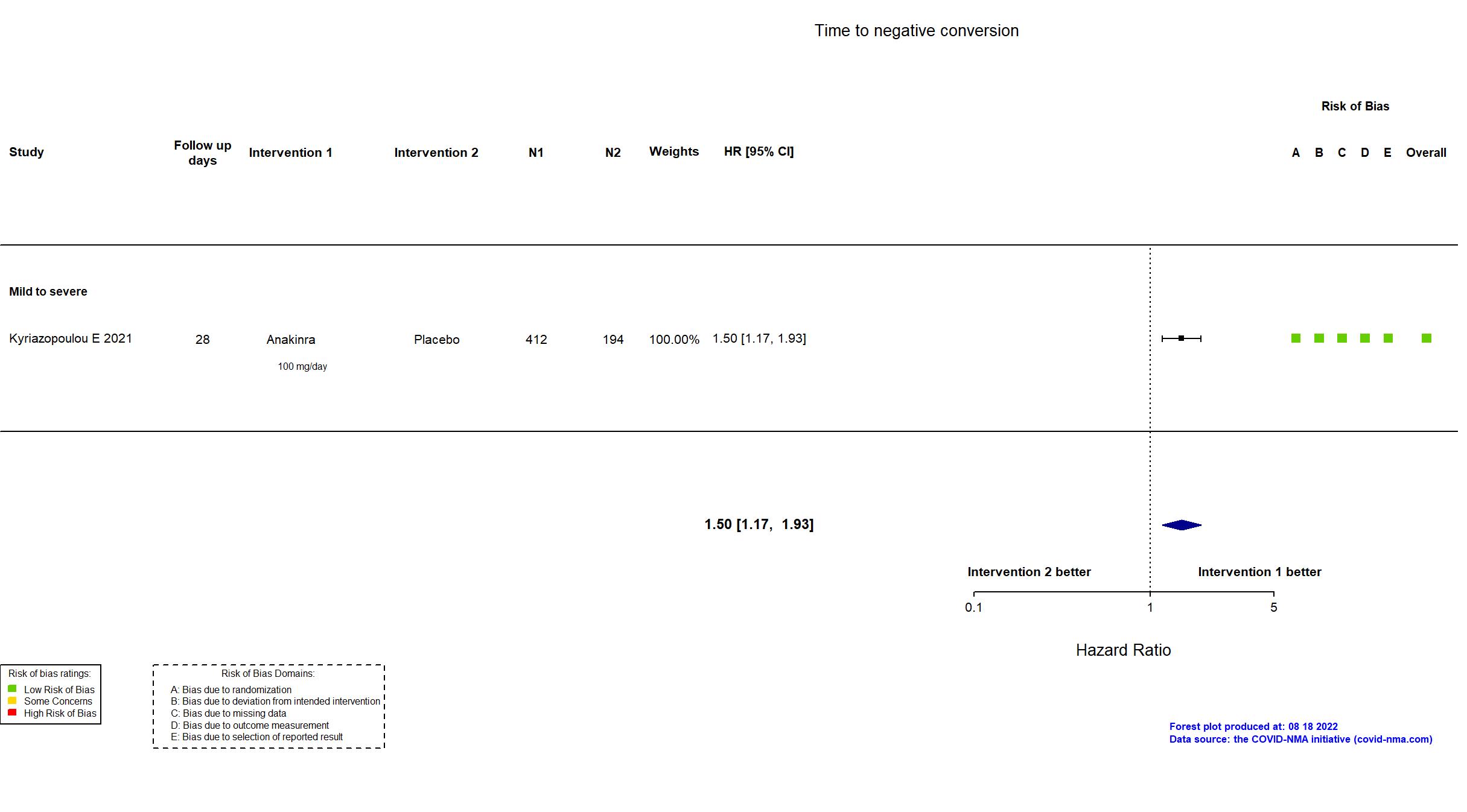
Trial NCT04680949; EudraCT 2020-005828-11
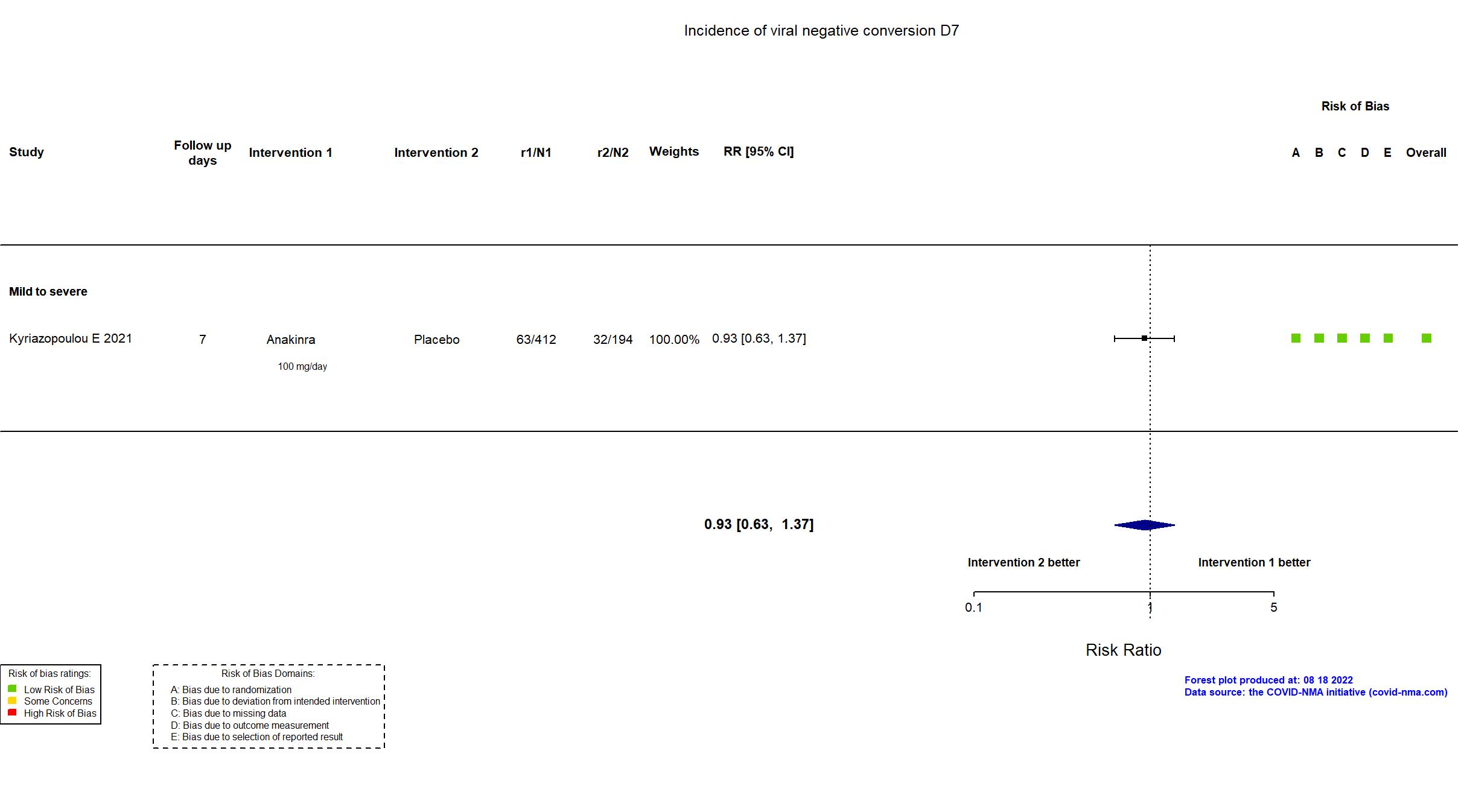
Publication SAVE-MORE - Kyriazopoulou E, Nat Med (2021) (published paper)
Dates: 2020-12-23 to 2021-03-31
Funding: Mixed (Hellenic Institute for the Study of Sepsis and Swedish Orphan Biovitrum AB (Sobi))
Conflict of interest: Yes
Trial NCT04341584
Publication CORIMUNO-ANA-1 - Mariette X, Lancet Respir Med (2021) (published paper)
Dates: 2020-04-08 to 2020-04-26
Funding: Public/non profit (The Ministry of Health, Programme Hospitalier de Recherche Clinique, Foundation for Medical Research, and AP-HP Foundation )
Conflict of interest: No